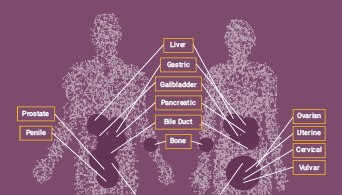
Inestabilidad de Microsatélites
El análisis de MSI mide funcionalmente la acumulación genómica de los errores de inserción o deleción (INDEL) causados por deficiencias en el sistema de reparación de apareamientos erróneos (dMMR, por sus siglas en inglés) que ocurre en ciertos tipos de tumores sólidos, y este cribado puede usarse para caracterizar mejor los tumores y guiar las opciones terapéuticas para los tipos de cáncer MSI-Alto (MSI-H). Se ha demostrado que los tumores con estado MSI-H responden a las terapias con inhibidores del punto de control inmunitario (ICI).
MSI ha sido empleado en el estudio del síndrome de Lynch o cáncer colorrectal hereditario no asociado a poliposis, que es una condición hereditaria que incrementa la probabilidad de presentar ciertos tipos cáncer.
¿Qué es?
La Importancia de MSI
Aprenda sobre los usos de MSI como biomarcador de referencia en oncología y como apoyo en la toma de decisiones sobre el tratamiento.
Métodos de detección
Compare los diferentes métodos empleados para analizar MSI y/o MMR.
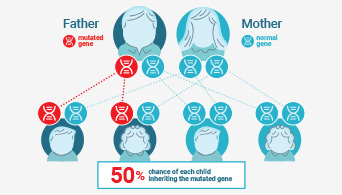
Understanding Lynch Syndrome
Learn more about the importance of determining Lynch status.
Interested in learning more about tools for MSI analysis?
Significance of MSI Status in Cancer Research and Diagnostics
Microsatellite instability (MSI) has been linked to cancer since 1993 when its association with Lynch syndrome, a hereditary nonpolyposis colon cancer, was first reported(1,2). Lynch syndrome is caused by mutations to the major DNA mismatch repair genes. In addition to colorectal cancer, Lynch syndrome is associated with several other cancers including endometrial, ovarian and stomach cancer. MSI analysis is used along with immunohistochemistry (IHC) as a screening test to determine if Lynch syndrome is likely. The results of these tests can indicate whether more specific genetic testing for Lynch syndrome should be performed.
The functional deficiency within one or more major DNA mismatch repair proteins (dMMR) that causes MSI also results in genetic instability and is closely related to the carcinogenicity of tumors(3). Tumors with dMMR demonstrate an increased number of mutations (hypermutation) and an increase in the predicted number of new antigens appearing because of these mutations(4,5). Therefore, tumors with high-frequency MSI (MSI-H) are more effective at priming an immune response. In some cancers, the presence of MSI predicts a good immunotherapeutic response, and can be used to inform treatment decisions(6).
Interest in MSI has exploded in recent years, driven by the discovery that its presence in tumor tissue can be predictive of a positive response to anti-PD-1 immunotherapies(4,5). Immune checkpoint inhibitor therapies targeting proteins such as PD-1 have revolutionized cancer treatment options, successfully treating certain cancers(2). When mistakes in DNA replication go uncorrected in cancer cells, they produce “foreign” proteins, called mutation-associated neoantigens (MANA), which can be detected by the immune system. Tumors expressing these proteins are effective at priming an immune response and are subsequently susceptible to immunotherapies, such as immune checkpoint inhibitor therapies.
For the most effective use of immunotherapy, it is important to identify accurate biomarkers that can be used to predict susceptibility. Some biomarkers, such as PD-1 expression, are accurate in predicting the response to immunotherapy in certain cancer types(7). MSI-H (as determined by PCR-based assays or NGS) or dMMR (as determined by IHC) are recognized as predictive biomarkers for these therapies. MSI status was the first biomarker approved for clinical use as a predictor of response to immunotherapy irrespective of the tumor type(7,8).
References:
- Boland, C.R. and Lynch, H.T. (2013) Fam. Cancer. 12, 45–57.
- Boland, P.M., Yurgelun, M.B. and Boland, C.R. (2018) CA Cancer J Clin. 68(3), 217–31.
- Baretti, M. and Le, D.T. (2018) Mismatch repair in cancer. Pharmacol. Therap. 189, 45–62.
- Le, D.T. et al. (2015) PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 372, 2509.
- Dudley, J.C., Lin, M.T., Le, D.T. and Eshleman, J.R. (2016) Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin. Cancer. Res. 22, 813.
- Kang, S., et al. (2018) Medicine (Baltimore). 297(9), e0019.
- Duffy, M.J. and Crown, J. (2019) Clin. Chem. 65, 1228–38.
- Li, K., et al. (2020) Cancer Cell International. 20(16).