GPCR Research for Drug Discovery
G protein-coupled receptors (GPCRs) represent one of the most important classes of drug targets. Discover how to measure the response along each step of the GPCR signaling cascade with easy-to-use, bioluminescence-based assays.
Overview
GPCR Signaling Pathways
Luciferase-based assays offer convenient, sensitive methods to measure GPCR activity for multiple signaling pathways.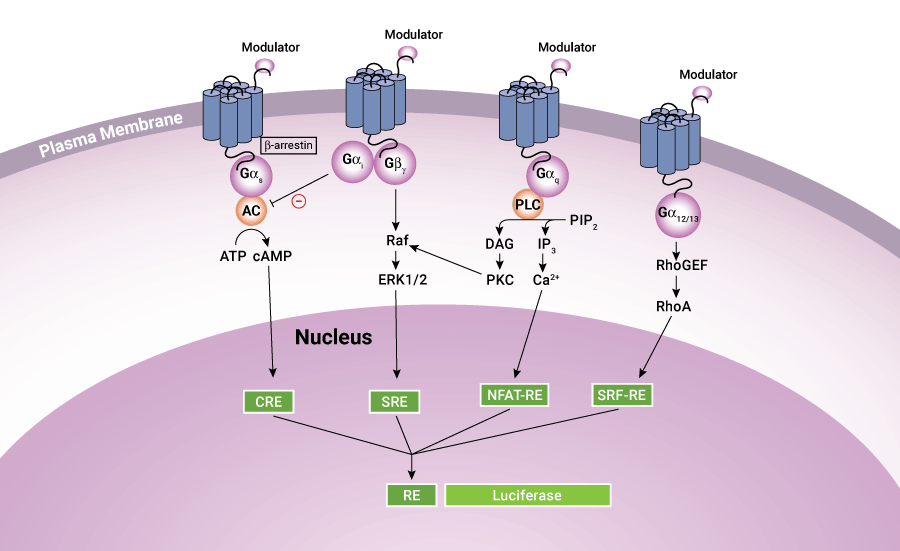
Case Study: University of Nottingham Medical School

Dr. Stephen Hill describes a novel approach, using NanoBRET® and NanoLuc® technologies, to study the effects of drugs on a GPCR that recognizes and responds to neurotransmitters.
Ligand Binding
Paper: An Integrated Approach toward NanoBRET® Tracers for Analysis of GPCR Ligand Engagement ›
GPCR Interactions
Interactions of activated GPCRs with intracellular proteins, such as G proteins, GPCR kinases and β-arrestins, are critical steps in G protein signaling, making these key areas of research for drug discovery. Sensitive bioluminescent assays enable measurement of these interactions in live cells to assess real-time kinetics in high-throughput compatible formats.
GPCR Internalization
Internalization of ligand activated GPCRs is an important step in regulating receptor signaling. The HiBiT tagging system makes it possible to develop simple, quantitative assays for receptor internalization and subsequent recycling that save time and eliminate the variability associated with antibody-based methods.
HiBiT-Tagged GPCR Internalization
- Assays on live cells for real-time kinetic analysis,
- No fixing, washes or antibodies required
- Total receptor measurement on the same cells
- Compatible with endogenously tagged receptors for measuring native biology

Isoproterenol-dependent internalization monitored with the Nano-Glo® HiBiT Extracellular Detection System. The HiBiT tag was introduced into the β2-AR gene in HEK293 cells using CRISPR gene editing.
Transcriptional Responses

cAMP Assays
Monitor cAMP production in response to the effects of test compounds on GPCRs with sensitive and easy-to-use cAMP-Glo™ and GloSensor™ cAMP assays.
cAMP-Glo™ Assay
- High sensitivity (30 fmol ± 5 SEM cAMP/well) and reproducibility (Z´>0.8)
- Measure intracellular cAMP concentrations in a single tube or high-throughput plate-based format
- Compatible with adherent, suspension, and frozen cells, and tissue extracts
- Bioluminescent assay, less prone to compound interference than fluorescence-based assays
- Extended half-life of the luminescent signal (>4 hours), allowing for batch-mode processing of multiple plates
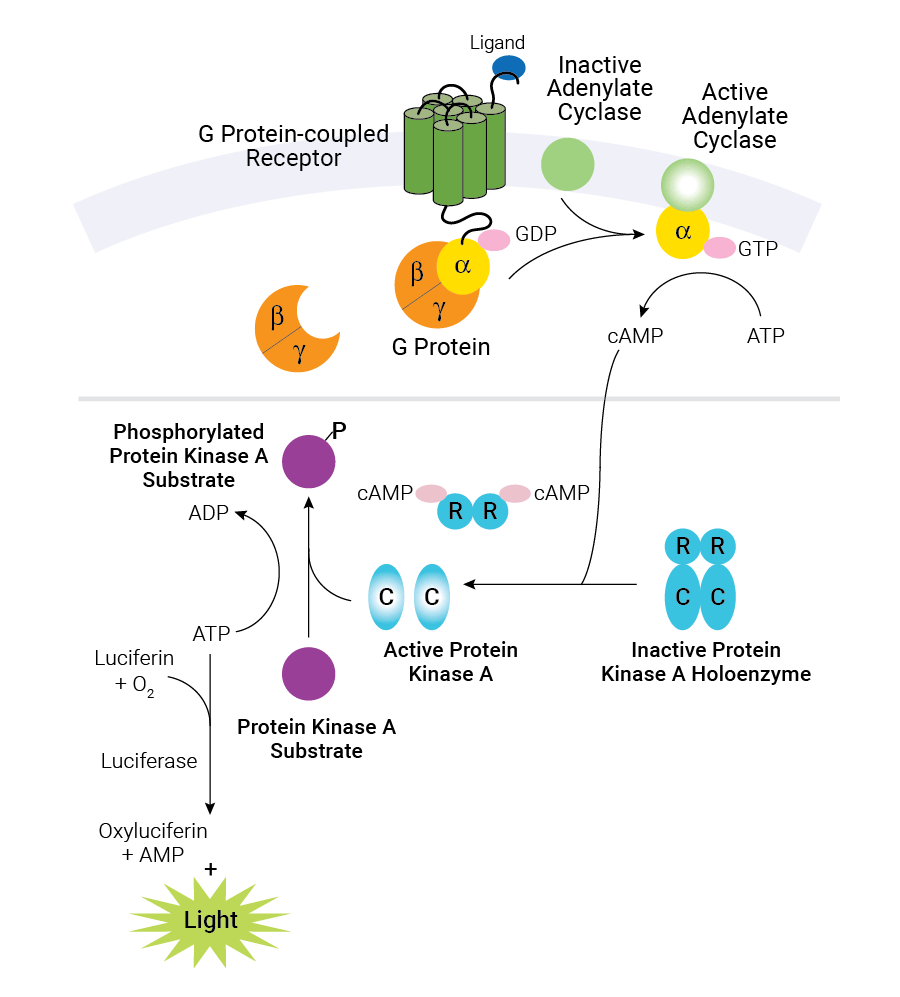
Schematic diagram of cAMP production in cells and the cAMP-Glo™ Max Assay. Binding of an extracellular ligand to its receptor alters the conformation of the associated heterotrimeric G protein, causing dissociation of the Gα and Gβγ subunits and initiating a cascade of cellular events. The alpha subunit is categorized into one of several groups: αs, αi/o, αq and α12/13. Gαs activates adenylate cyclase, while Gαi/o inhibits adenylate cyclase activity. The cAMP-Glo™ Max Assay is depicted in the shaded box. As the concentration of cAMP increases, cAMP binds to protein kinase A, and the regulatory subunits undergo a conformational change to release the catalytic subunits. The free catalytic subunits then catalyze the transfer of the terminal phosphate of ATP to a protein kinase A substrate, consuming ATP in the process. The level of remaining ATP is determined using the luciferase-based Kinase-Glo® Reagent. Luminescence is inversely proportional to cAMP levels. Thus, as cAMP concentration increases, luminescence decreases.
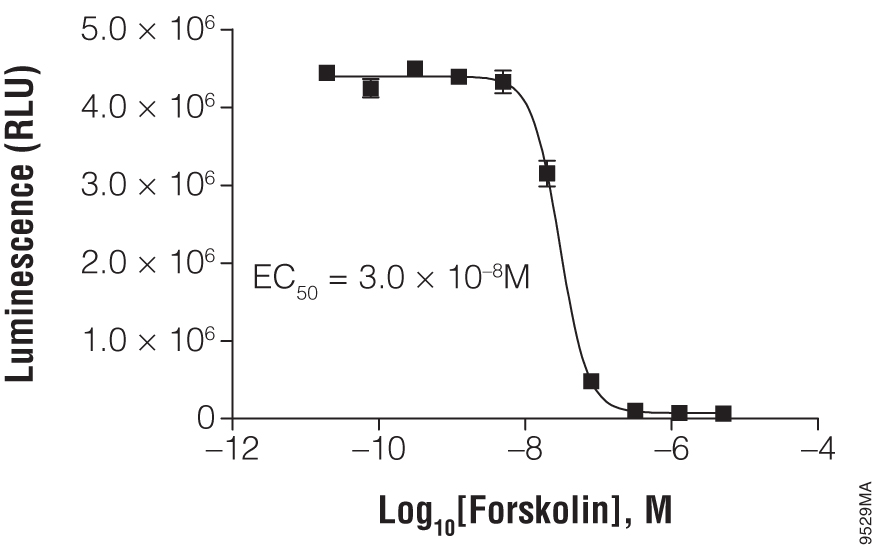
Titration of forskolin using the suspension cell line HEK293. In a white, clear-bottom, 384-well plate, 2,000 HEK293 cells were exposed to the indicated concentration of forskolin. The cAMP-Glo™ Max Assay was performed as described in the technical manual. Each point represents eight data points; the error bars represent the standard deviation. Data analysis was performed with GraphPad Prism® software using a sigmoidal dose-response (variable slope) equation.
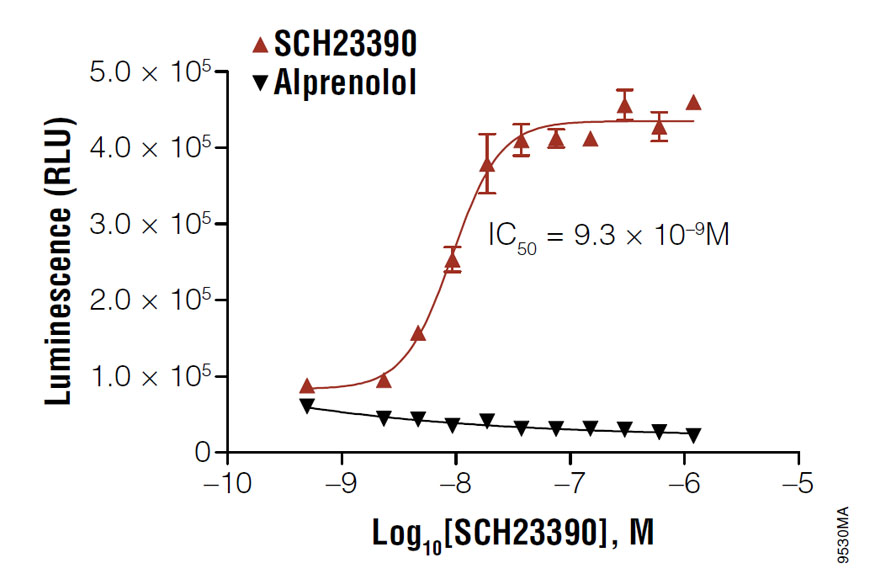
Determining the IC50 value of SCH23390 in D1 receptor-expressing HEK293 cells. Cells were resuspended in Induction Buffer and 2,000 cells were added to each well of a white solid 384-well plate. Cells were treated with the indicated amount of antagonist, SCH23390, in the presence of 100nM agonist, SKF38393. In the negative control reactions, SCH23390 was replaced with alprenolol. The cAMP-Glo™ Max Assay was performed as described in the technical manual. Data analysis was performed with GraphPad Prism® software using a sigmoidal dose-response (variable slope) equation.
Live-Cell GloSensor™ cAMP Assay
- Real-time detection of cAMP in live cells
- Broad dynamic range, showing up to 500-fold changes in light output
- Extreme sensitivity allowing detection of Gi-coupled receptor activation or inverse agonist activity in the absence of artificial stimulation by compounds such as forskolin
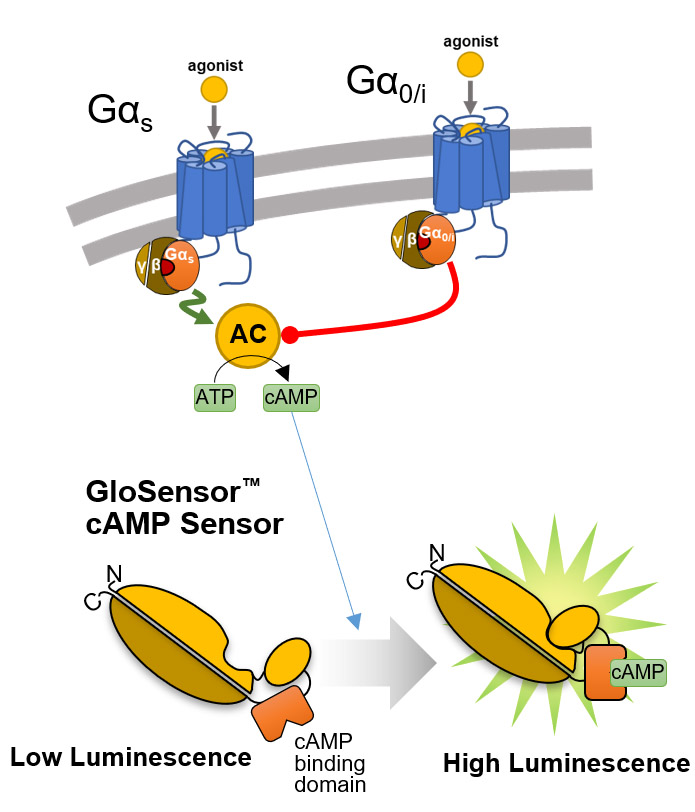
Overview of the GloSensor™ cAMP Assay. Genetically encoded biosensor variants contain cAMP binding domains fused to mutant forms of Photinus pyralis luciferase. Upon binding to cAMP, conformational changes occur that promote large increases in light output.
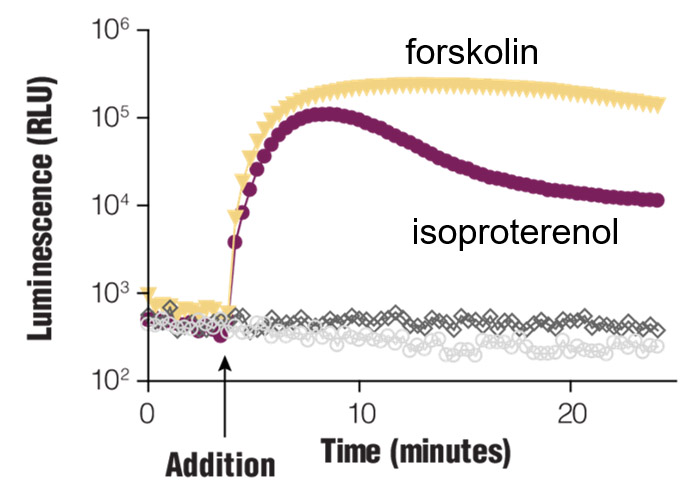
Real-time Gαs monitoring. HEK293 cells were transiently transfected with pGloSensor™-22F cAMP Plasmid and treated with compounds as shown.
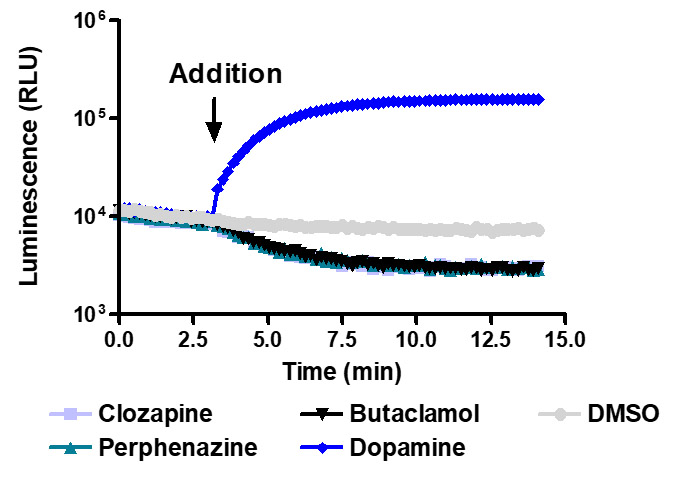
Sensitive enough to detect inverse agonists without forskolin. HEK293 cells expressing Dopamine D1 receptor were transiently transfected with pGloSensor™-22F cAMP Plasmid and treated with 10µM compounds at 28°C.
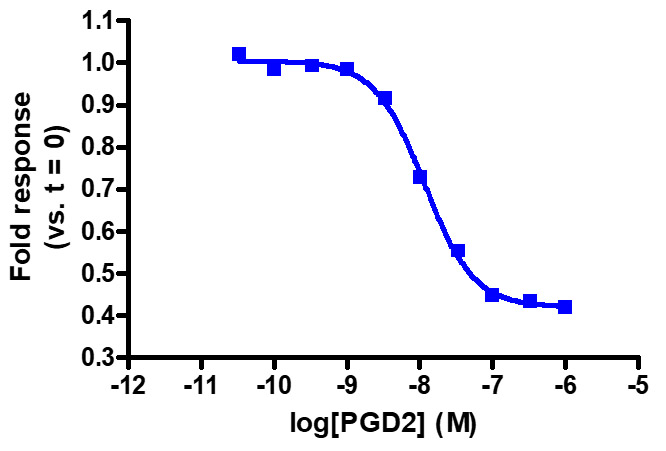
Sensitive enough to detect Gαi activity without forskolin. HEK293 cells expressing GPR44 were transiently transfected with pGloSensor™-22F cAMP Plasmid and treated with prostaglandin D2 (PGD2). Note that forskolin pretreatment increases the fold response (not shown).
GPCR Research Resources

Lighting Up GPCR Research with Bioluminescent Tagging
A summary of a research publication that used the HiBiT assay to measure surface expression of orexin and prokineticin receptors.

A NanoBRET® Biosensor for GPCR:G protein Interaction with the Kinetics and Temporal Resolution of Patch Clamping
Review of a study to detect signaling through Gα12/13 proteins, which had previously proved intractable, using NanoBRET® technology.

Measuring cAMP Levels and Cytotoxicity in a Single Plate Well
Multiplexing of a fluorescent real-time cytotoxicity assay with a bioluminescent cAMP assay, to examine cytotoxic effects through coupling of GPCRs with adenylate cyclase activity.