Microsatellite Instability Resource Center
Microsatellite Instability (MSI) is the accumulation of insertion or deletion errors at microsatellite repeat sequences in cancerous cells as a result of a functional deficiency within one or more major DNA mismatch repair proteins (dMMR). Mononucleotide (homopolymer) repeat microsatellite sequences found throughout the genome are particularly sensitive to transcription errors. Thus, high frequency microsatellite instability (MSI-H) is considered a marker for the presence of mutations in, or methylation silencing of, certain major DNA MMR genes.
Historically MSI has been used to screen for Lynch syndrome, a dominant hereditary cancer propensity. Recently, MSI status has been rediscovered as a biomarker for immunotherapeutic response, making MSI status an increasingly relevant tool for genetics and immuno-oncology applications.
What is MSI?
Importance of MSI Status
Learn the ways MSI status is used as a valuable biomarker for cancer risk and to inform treatment decisions.
Methods of Detection
Compare the different methods used to measure MSI and MMR status.
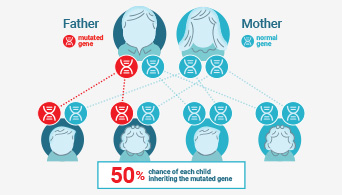
Understanding Lynch Syndrome
Learn more about the importance of determining Lynch status.
Interested in learning more about tools for MSI analysis?
Significance of MSI Status in Cancer Research and Diagnostics
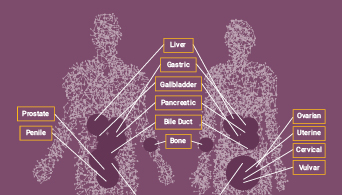
Microsatellite instability (MSI) has been linked to cancer since 1993 when its association with Lynch syndrome, a hereditary nonpolyposis colon cancer, was first reported(1,2). Lynch syndrome is caused by mutations to the major DNA mismatch repair genes. In addition to colorectal cancer, Lynch syndrome is associated with several other cancers including endometrial, ovarian and stomach cancer. MSI analysis is used along with immunohistochemistry (IHC) as a screening test to determine if Lynch syndrome is likely. The results of these tests can indicate whether more specific genetic testing for Lynch syndrome should be performed.
The functional deficiency within one or more major DNA mismatch repair proteins (dMMR) that causes MSI also results in genetic instability and is closely related to the carcinogenicity of tumors(3). Tumors with dMMR demonstrate an increased number of mutations (hypermutation) and an increase in the predicted number of new antigens appearing because of these mutations(4,5). Therefore, tumors with high-frequency MSI (MSI-H) are more effective at priming an immune response. In some cancers, the presence of MSI predicts a good immunotherapeutic response, and can be used to inform treatment decisions(6).
Interest in MSI has exploded in recent years, driven by the discovery that its presence in tumor tissue can be predictive of a positive response to anti-PD-1 immunotherapies(4,5). Immune checkpoint inhibitor therapies targeting proteins such as PD-1 have revolutionized cancer treatment options, successfully treating certain cancers(2). When mistakes in DNA replication go uncorrected in cancer cells, they produce “foreign” proteins, called mutation-associated neoantigens (MANA), which can be detected by the immune system. Tumors expressing these proteins are effective at priming an immune response and are subsequently susceptible to immunotherapies, such as immune checkpoint inhibitor therapies.
For the most effective use of immunotherapy, it is important to identify accurate biomarkers that can be used to predict susceptibility. Some biomarkers, such as PD-1 expression, are accurate in predicting the response to immunotherapy in certain cancer types(7). MSI-H (as determined by PCR-based assays or NGS) or dMMR (as determined by IHC) are recognized as predictive biomarkers for these therapies. MSI status was the first biomarker approved for clinical use as a predictor of response to immunotherapy irrespective of the tumor type(7,8).
References:
- Boland, C.R. and Lynch, H.T. (2013) Fam. Cancer. 12, 45–57.
- Boland, P.M., Yurgelun, M.B. and Boland, C.R. (2018) CA Cancer J Clin. 68(3), 217–31.
- Baretti, M. and Le, D.T. (2018) Mismatch repair in cancer. Pharmacol. Therap. 189, 45–62.
- Le, D.T. et al. (2015) PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 372, 2509.
- Dudley, J.C., Lin, M.T., Le, D.T. and Eshleman, J.R. (2016) Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin. Cancer. Res. 22, 813.
- Kang, S., et al. (2018) Medicine (Baltimore). 297(9), e0019.
- Duffy, M.J. and Crown, J. (2019) Clin. Chem. 65, 1228–38.
- Li, K., et al. (2020) Cancer Cell International. 20(16).