Protein Localization, Imaging, Trafficking and Turnover
Whether you want to label proteins on the cell surface or inside the cell, specific, irreversible fluorescent HaloTag® ligands can help you locate and track the movement of your protein of interest.
Determine the Intracellular Location of Your Protein of Interest
Looking to label intracellular proteins? Choose from fluorescent HaloTag® ligands that can enter into the cells to find the HaloTag® fusion protein of interest. The HaloTag® protein forms a stable covalent bond with the HaloTag® ligand under physiological conditions, offering high labeling specificity and a stable complex. Follow the cellular fate of your protein after labeling it once or use a pulse-chase option by labeling with a second fluorescent label after a period of time. Pulse-chase labeling can show protein trafficking or turnover over time.
Fluorescent Labeling of HaloTag® Fusion Proteins

Cells are imaged using proteins fused with HaloTag® protein and fluorescent HaloTag® ligands. You can image cells with or without washing to determine subcellular location.
Cellular Imaging Using Fluorescent HaloTag® Ligands
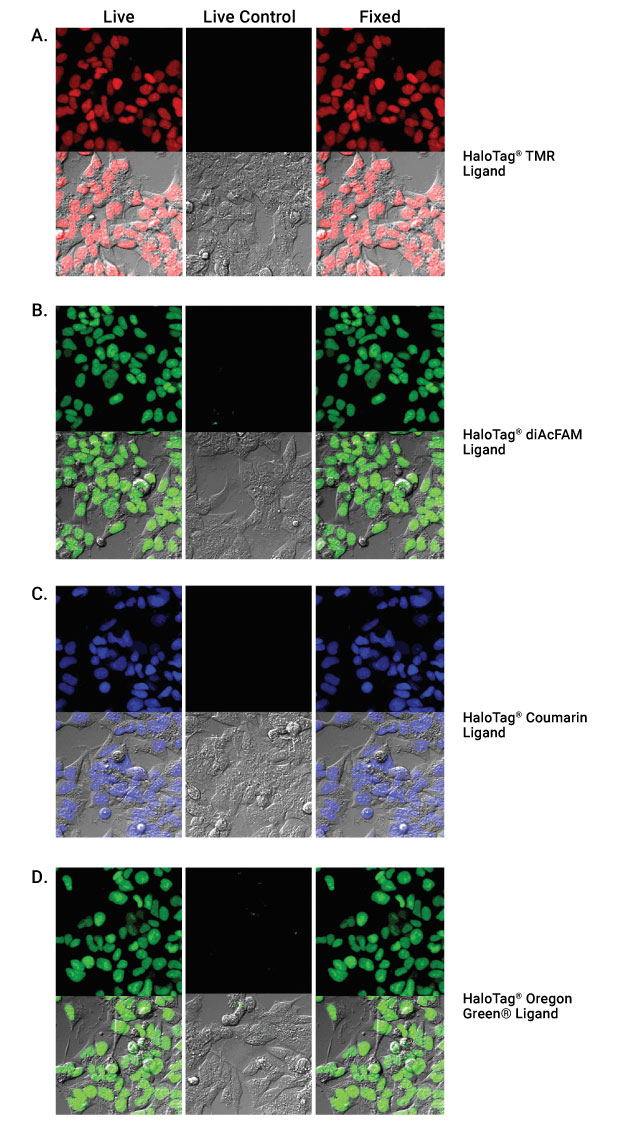
HaloTag® ligand labeling of live cells followed by fixation. HEK293 cells stably transfected with the HaloTag® gene fused to three copies of a nuclear localization sequence and nontransfected HEK293 controls labeled with HaloTag® TMR (Panel A), HaloTag® diAcFAM (Panel B), HaloTag® Coumarin (Panel C) or HaloTag® Oregon Green® Ligand (Panel D).
Investigate Membrane Protein Localization
Examine receptor trafficking by labeling your membrane protein of interest fused with HaloTag® protein with one of the fluorescent ligands that cannot enter the cell. The covalent bond between the HaloTag® reporter protein and the ligand means even under the denaturing conditions required for fixing cells, you can determine if your protein is still present in the membrane or if there was turnover or internalization. These cell impermeant HaloTag® ligands are covalently bonded to the HaloTag® protein, meaning you can analyze the protein under stringent conditions.
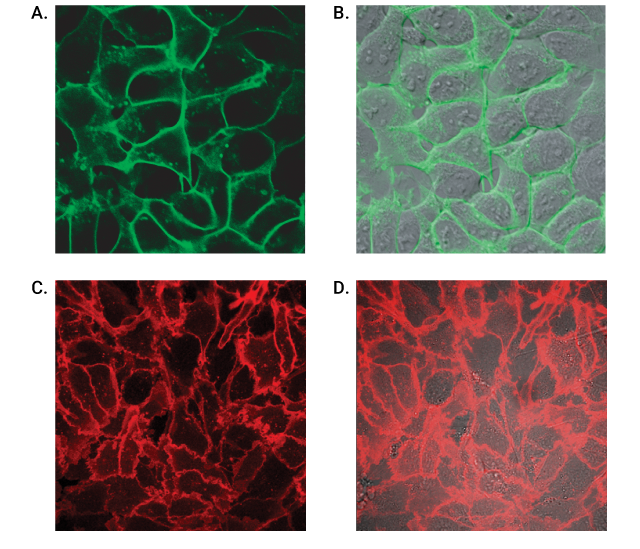
Live-cell labeling of cell surface HaloTag® fusion proteins. Panels A and B show HEK293 cells stably expressing HaloTag®-ECS (ExtraCellular Surface; comprised of a signal sequence and single transmembrane domain of b1-integrin) fusion protein labeled with HaloTag® Alexa Fluor® 488 Ligand and then imaged. Panels C and D show U2OS cells expressing HaloTag®-EDG1 (GPCR receptor) fusion labeled with Alexa Fluor® 660 Ligand and then imaged. Confocal images show fluorescence (Panels A and C) is restricted to the cell surface, also seen clearly in the panels with the DIC/fluorescence overlay (Panel B and D). Images were generated on an Olympus FV500 confocal microscope using the appropriate filter sets.
Multiplex Using Immunocytochemistry
Using the Anti-HaloTag® Polyclonal Antibody with a secondary antibody makes it possible to colabel HaloTag® fusion proteins with a fluorescent ligand. Confirm that your protein was specifically labeled by the HaloTag® ligand in vivo especially when the secondary antibody uses a spectrally distinct fluorophore for immunocytochemistry (ICC). The HaloTag® ligand can be used with live cells and then the cells are fixed for ICC using the Anti-HaloTag® pAb. ICC labeling is specific to the HaloTag® protein, reducing background staining.
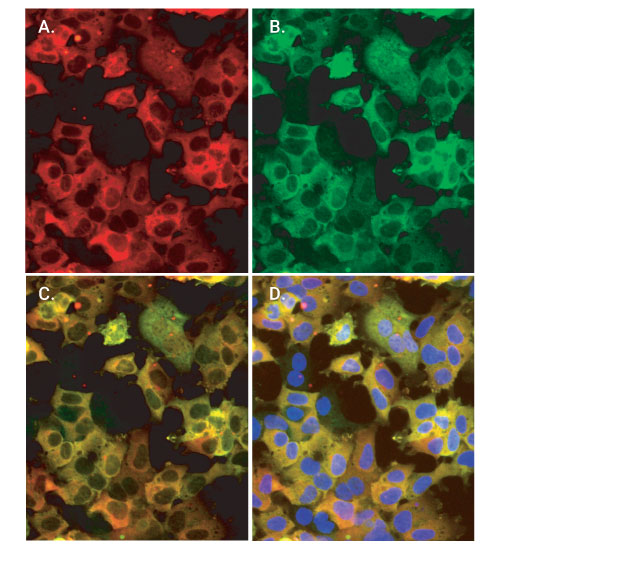
Colabeling of HaloTag®-p65 fusion protein with HaloTag® TMR ligand and the Anti-HaloTag® pAb. Cells were labeled with 5µM HaloTag® TMR Ligand for 15 minutes, gently washed and then incubated with 2µg/ml AlexaFluor® 488-conjugated anti-rabbit IgG (Invitrogen). Finally, cells were counterstained with DAPI (Vector Laboratories). Images were collected on the Olympus IX81 epi-fluorescent microscope with filter sets appropriate for rhodamine and AlexaFluor® 488. Panel A. Cytoplasmic (red) labeling of HEK293-p65-HT2 cells by HaloTag® TMR Ligand. Panel B. Cytoplasmic (green) labeling by Anti-HaloTag® pAb. Panel C. Colocalization of ligand and antibody binding activities. Panel D. Merger of red and green fluorescence with counterstaining of the nucleus by DAPI (blue). Arrows denote rare cells that show little or no expression of HaloTag®-p65.
Additional HaloTag® Resources
Find Your HaloTag®-Gene Fusion Vector
Start imaging faster by searching our Find My Gene tool for a ready made HaloTag® vector with your gene of interest.
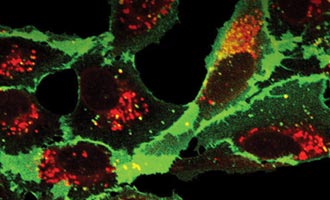
Localize Proteins in Live and Fixed Cells
Learn how the HaloTag® Interchangeable Labeling Technology can locate where in the cell a protein is in this article.
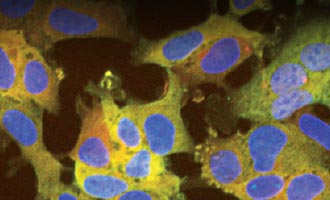
Confirm Ligand Specificity Using ICC
Read how a polyclonal HaloTag® antibody can be used in immunocytochemistry for colabeling experiments in this article.
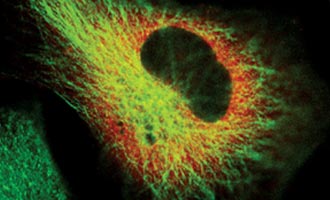
Get Started Imaging Protein Movement and Localization
View this webinar to learn how the HaloTag® Interchangeable Labeling Technology can tag and locate a cellular protein.
Analyze Your Protein with One Genetic Construct
This Bioconjugate Chemistry review article highlights HaloTag® Technology as a versatile platform for exploring protein function.