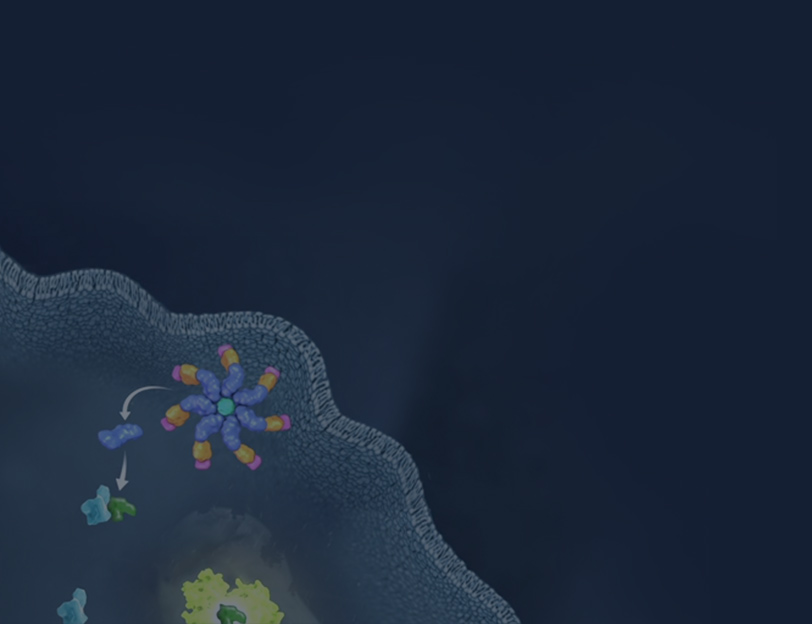
“Lumitizing” an ELISA
Introduction
The Lumit® platform employs NanoLuc® Binary Technology (NanoBiT®) to deliver bioluminescent immunoassays for fast and easy analyte detection (1). NanoBiT® Binary Technology consists of two subunits, Small BiT (SmBiT; 1.3kDa) and Large BiT (LgBiT; 18kDa), that have been individually optimized for stability and minimal spontaneous association (Kd=190μM; 2). When antibodies labeled with SmBiT and LgBiT subunits, respectively, bind to a shared target, they bring the complementary NanoBiT® subunits into proximity and promote reconstitution of NanoBiT® Luciferase. This, in the presence of Lumit® Detection Substrate, generates a luminescent signal proportional to the level of analyte. Lumit® Immunoassays offer several advantages, including exceptionally high signal-to-background ratios, linear dynamic ranges exceeding three logs (significantly mitigating the need for sample dilution), a rapid execution time (<2 hours), and a straightforward add-and-read protocol with a stable luminescent readout suitable for high-throughput screening applications.
The Lumit® platform offers both ready-to-use assays (e.g., Lumit® cytokine immunoassays) and do-it-yourself (DIY) tools for researchers to create their own Lumit® Immunoassays for targets of interest. High-quality antibodies are essential when developing new Lumit® Immunoassays. Using ELISA-validated antibody pairs is not obligatory or a guarantee of Lumit® Immunoassay performance. However, using ELISA-validated antibody pairs will help generate SmBiT- and LgBiT-labeled antibodies that have a high probability of simultaneous binding to a target. Given the ease of antibody testing with Lumit® Technology and the substantial performance and workflow benefits of a positive assay result, adapting an ELISA into a Lumit® format (or “Lumitizing” an assay) can yield significant productivity gains. In this guidance document, we provide a clear process for generating NanoBiT®-labeled antibody pairs, and then evaluating and optimizing their performance in the preferred Lumit® Immunoassay format.
Target Considerations
Lumit® Immunoassays have been effectively developed for a broad range of protein targets, from very small (<8kDa) to very large (>300kDa). This includes secreted and intracellular targets, viral particles and analytes in human serum samples. However, the use of an antibody pair validated in ELISA format does not automatically ensure success in the Lumit® format. This is partly due to the Lumit® Immunoassay’s requirement for NanoBiT®-labeled antibodies to bind to their shared target in a manner that supports unhindered reconstitution of NanoBiT® Luciferase. Consequently, the likelihood of success may be reduced for larger targets or those in complex structures, like some intracellular targets, depending on the relative locations of antibody binding sites. Additionally, the homogeneous nature of the Lumit® Immunoassay can lead to potential interference from substances in the sample matrix, an issue somewhat less pronounced in the heterogeneous ELISA format. Despite these challenges, the rapid testing of ELISA-validated antibodies in the Lumit® format makes the effort worthwhile due to the significant advantages of a successful adaptation.
Workflow for “Lumitizing” an ELISA
Although there is no certainty that antibodies validated for ELISA will work in a Lumit® format, the testing process is straightforward and can unlock the advantages of a Lumit® Immunoassay. The essential steps include:
- Selecting Primary Antibodies
- Choosing an Assay Standard or Positive Control
- Labeling Antibodies with SmBiT and LgBiT Subunits
- Analyzing Labeled Antibodies via Nonreducing SDS-PAGE
- Screening Labeled Antibodies for Target Detection
- Assessing Sensitivity and Linearity of Assay Performance
- Verifying Assay Performance with a Relevant Model
1. Selecting Primary Antibodies
In this document, we focus on using primary antibodies labeled with NanoBiT® Luciferase subunits for Lumit® Immunoassays. This “direct” approach allows the use of primary antibody combinations from the same or different species. Thus, when converting ELISA antibodies to Lumit® Immunoassays, it’s acceptable to use antibodies from the same species (e.g., two rabbit mAbs). Note that if the primary antibodies are from complementary species (e.g., rabbit and mouse), their unlabeled versions can be rapidly tested with NanoBiT®-labeled secondary antibodies in an “indirect” assay setup.
Key considerations for selecting primary antibodies to label for Lumit® Immunoassays include:
- Unconjugated Antibodies: For direct configuration, antibodies must be available in an unconjugated form for NanoBiT® subunit labeling.
- Type of Antibodies: Both monoclonal (mAb) and polyclonal (pAb) antibodies are suitable, provided they have high affinity and specificity for the target.
- Purity: High purity (e.g., Protein A affinity purified) is ideal.
- Formulation Considerations: The antibody formulation should not contain components that interfere with amine-directed labeling (e.g., Tris, Glycine, azide, BSA, glycerol, sucrose, trehalose). However, small molecule additives and buffers like Tris, azide, or sucrose, can be removed via buffer exchange using desalting columns before labeling.
- Concentration for Labeling: A starting concentration of 1mg/ml is recommended for optimal labeling, although concentrations ranging from 0.5–5mg/ml can also be successful.
2. Choosing an Assay Standard or Positive Control
In developing a Lumit® Immunoassay, choosing an appropriate standard, such as a recombinant protein or positive control, is crucial for evaluating assay effectiveness and for quantitative applications to measure analyte concentration. When converting an ELISA to a Lumit® Immunoassay, the ELISA standard, often a recombinant protein, is typically suitable. However, it’s important to note that some components in ELISA kit standards might interfere with the Lumit® Immunoassay homogeneous chemistry. In such cases, alternative standards should be considered, ensuring they contain the epitopes recognized by the primary antibodies. For targets such as phosphorylated signaling proteins where high-quality standards are unavailable, Lumit® Immunoassay performance can be evaluated using cell samples combined with pharmacological or genetic tools (e.g., pathway modulators or wildtype versus knockout cells).
3. Labeling Antibodies with SmBiT and LgBiT Subunits
The Lumit® Immunoassay Labeling Kit Technical Manual, #TM602, offers detailed instructions for labeling antibodies (Ab1 and Ab2) with NanoBiT®. Luciferase subunits, SmBiT and LgBiT. Below is a summarized workflow control:
- Preparation: Check Ab1 and Ab2 formulations for interfering substances. Use a desalting column (e.g., Zeba Spin Desalting Columns) for buffer exchange if necessary. (Can be combined with the pH adjustment step).
- pH Adjustment: Bring Ab1 and Ab2 solutions to pH 8.5, either by buffer exchange into 10mM sodium bicarbonate (NaHCO3) buffer or by adding 1/10th volume of 1M NaHCO3 buffer directly.
- Activating with HaloTag® Ligand: React Ab1 and Ab2 with HaloTag® Ligand for 90 minutes at room temperature, using a 20-fold molar excess of ligand (up to 50-fold for Ab concentrations around 0.5mg/ml). This step targets the free amine groups on the antibodies. After this, remove unbound HaloTag® Ligand with desalting columns, exchanging into PBS buffer, pH 7.
- Attaching NanoBiT® Subunits: Add 1/200th volume of 10% IGEPAL to the antibodies activated with HaloTag® Ligand to reduce precipitation. Split each activated antibody into two aliquots. Add 4-fold molar excess of HaloTag® -SmBiT to one aliquot and HaloTag® -LgBiT to the other. Incubate for 16–20 hours at 4°C with gentle mixing. This results in four labeled antibodies: Ab1-SmBiT, Ab1-LgBiT, Ab2-SmBiT, and Ab2-LgBiT. Optionally, assay performance may be improved by removing unreacted HaloTag® -SmBiT and HaloTag® -LgBiT using Magne® HaloTag® Beads.
4. Labeling Antibodies with SmBiT and LgBiT Subunits
To ensure quality labeling, analyze each antibody labeled with NanoBiT® subunits using non-reducing SDS-PAGE. Successfully labeled antibodies will display as protein ladders on the stained gel, with each band representing the addition of another NanoBiT® subunit. Note that heating the antibody sample in SDS loading buffer to 75°C for 5 minutes before loading onto the gel can improve the resolution between unlabeled and labeled antibody species. Deviating from this heating protocol may result in gel artifacts.
5. Screening of Labeled Antibodies for Target Detection
After obtaining NanoBiT®-labeled antibodies, their effectiveness in target detection can be tested through complementary pairings: Ab1-SmBiT with Ab2-LgBiT, and the opposite configuration, Ab1-LgBiT with Ab2-SmBiT. Each pairing should be tested against a reasonable concentration of standard, as well as in the absence of standard, to allow signal-to-background (S/B) determinations. Given that only two antibody pairings are to be tested, evaluate them across a range of concentrations to determine: a) the primary antibodies’ effectiveness in the Lumit® format; b) the preferred antibody labeling configuration; and c) the optimal concentration of each labeled antibody for the best S/B ratio while ensuring adequate assay signal.
6. Assessing Linearity and Sensitivity of Assay Performance
Now you are ready to assess linearity and sensitivity of your candidate Lumit® Immunoassay. Using the optimal concentrations of SmBiT- and LgBiT-labeled antibodies for the selected pair, obtain assay signal across a wide range of recombinant standard concentrations in the intended sample matrix (e.g., complete cell culture medium). Be sure to include a no-standard control for background determination. While a plot of S/B versus analyte concentration provides a useful assessment of assay performance, to more effectively assess assay linearity, plot background subtracted luminescence values on a log-log scale against analyte concentration. Subsequently, fit the data using the power equation (y = mxe), where exponent values near one indicate high linearity. Lumit® Immunoassays typically show linear ranges over three or more logs of analyte concentration, often surpassing the ELISA format using the same antibodies and thereby reducing the need for sample dilution. However, be aware of the “hook effect” where luminescence signals may level off or even decrease when exceeding the upper limit of assay linearity. If there is any doubt about whether analyte levels for an undiluted sample are within the assay’s linear range, we advise performing further assessments, such as assay of serial twofold dilutions of the sample.
“Lumitizing” an ELISA Example: Mouse IL-2
Lumit® Immunoassays rely on NanoBiT® Luciferase Technology. A cartoon rendition of antibodies labeled with SmBiT and LgBiT subunits is shown in Figure 1. Upon antibody binding to target analyte, the NanoBiT® subunits are brought into close proximity and form an active luciferase enzyme. This generates a luminescent signal in the presence of the luciferase substrate. To demonstrate the process for “Lumitizing” an ELISA, we will highlight an example using mouse IL-2 (mIL-2) as the target of interest.



Figure 1. Confirmation of antibody labeling by nonreducing SDS-PAGE gel. Capture and Detector monoclonal antibodies to mouse IL-2 (Abcam, Cat.#s ab242470 and ab242721, respectively) were labeled with HaloTag®-SmBiT and HaloTag®-LgBiT subunits. Samples from the labeling process were resolved as follows: lanes 1–2, HaloTag®-Ligand activated antibodies; lanes 3–4, HaloTag®-BiTs; lanes 5–8, antibodies after conjugation with HaloTag®-BiTs; and lanes 9–12, antibody-BiT conjugates after clean-up with Magne® HaloTag® Beads to remove most of the unbound HaloTag®-BiTs.
Following the process described in Section 3: Labeling Antibodies with SmBiT and LgBiT Subunits, we activated ELISA-validated Capture and Detector antibodies for mouse IL-2 with HaloTag® Ligand. Next, we labeled the antibodies with HaloTag®-SmBiT and HaloTag®-LgBiT to generate mAb-SmBiT and mAb-LgBiT conjugates for each antibody. Figure 1 shows the protein stain of these labeled antibodies following resolution with nonreducing SDS-PAGE. Labeled antibodies exhibit multiple higher molecular weight bands indicating a distribution of protein with one, two, or more HaloTag®-BiT subunits, concomitant with the expected decrease in unlabeled antibody. Unreacted HaloTag®-SmBiT and HaloTag®-LgBiT do not interact due to weak affinity (190μM) but can be removed if desired and may decrease assay background.

Figure 2. Screening of labeled antibodies for target detection. Alternative configurations of the complementary labeled anti-mouse IL-2 antibodies, referred to as Antibody Pair 1 and Antibody Pair 2, were used to detect recombinant mouse IL-2 (mIL-2) at a range of antibody concentrations. Each antibody combination was tested in the presence and absence of a fixed concentration of target analyte (100nM mIL-2). Luminescence readings (RLU) were obtained using a GloMax® Discover Microplate Reader.
Once we confirmed NanoBiT® labeling of Capture and Detector mAbs, we tested the labeled antibodies as described in Section 5: Screening of Labeled Antibodies for Target Detection. Figure 2 shows twofold serial dilutions of Antibody Pair 1, comprised of Capture mAb-SmBiT and complementary Detector mAb-LgBiT, combined in a white 96-well assay plate. In a similar manner, we also evaluated Antibody Pair 2, comprising the alternative pairing of Capture mAb-LgBiT with Detector mAb-SmBiT. We tested all combinations in culture medium in the presence and absence of analyte (100ng/ml recombinant mouse IL-2) to enable signal and background determinations, respectively. In this example, we performed the nonlytic Lumit® Immunoassays in cell culture medium (DMEM + 10% heat-inactivated FBS) using Lumit® Detection Reagent B. Luminescence readings were obtained with a GloMax® Discover Multiplate Reader. The data show effective detection of mouse IL-2 with generally low assay background in the absence of analyte.

Figure 3. Signal/background analysis for selection of optimal antibody pairing. Calculated S/B ratios are shown for Antibody Pair 1 and Antibody Pair 2 configurations. The selection of the preferred labeling configuration, as well as the optimal concentration of each antibody in the pair, can be made based on highest S/B ratios observed (boxed cells) and overall signal strength. S/B = (RLU with analyte)/(RLU without analyte).
We used obtained luminescence readings to calculate the signal-to-background (S/B) ratios for each pairing of labeled antibodies tested (Figure 3). While assay performance can sometimes be significantly impacted by which antibody in a pair receives which NanoBiT® subunit, in this example, both configurations of labeled antibody pairs provided excellent S/B ratios. Pair 1 yielded a maximal S/B of 1327 with 0.25μg/ml Capture mAb-SmBiT and 0.25μg/ml Detector mAb-LgBiT. The alternative configuration, Pair 2, generated a similar maximal S/B of 1270, albeit at the higher concentration of 0.5μg/ml for both Capture mAb-LgBiT and Detector mAb-SmBiT.
A

B

Figure 4. Lumit® titration assay for antibody pair 1. Graphs show Lumit® titration curves fitted to a four-parameter sigmoidal equation, for the antibody configuration pair 2: 0.5µg/ml Detector Ab-SmBiT, 0.5µg/ml Capture Ab-LgBiT. Panel A. S/B versus recombinant mouse IL-2 concentration. Panel B. Power equation fit of background-subtracted luminescence values plotted against recombinant mouse IL-2 concentration.
Next, we more rigorously evaluated assay performance by following the guidance described in Section 6: Assessing Linearity and Sensitivity of Assay Performance. The example data (Figure 4) were obtained using optimal concentrations of Antibody Pair 2, which provided similar maximal S/B as Pair 1 but with a somewhat brighter signal. A plot of S/B versus recombinant mouse IL-2 concentration (Figure 4, Panel A) is consistent with a strong assay window over a wide range of analyte concentrations. The Power equation fit of background-subtracted luminescence values plotted against analyte concentration (Figure 4, Panel B) demonstrated excellent linearity over more than three logs of mouse IL-2 concentration. For subsequent routine application of the assay, we would typical use a defined seven-point serial dilution of standard to quantitate analyte levels in tested samples. (For useful guidance on quantitation of unknowns using Lumit® immunoassays, see Lumit® Cytokine Assays: Interpolating Data with the GloMax® Discover; 3).
Additional “Lumitizing” ELISA Examples
As discussed above, application of Lumit® Technology to an ELISA-validated antibody pair can deliver excellent Lumit® Immunoassay performance. However, success in “Lumitizing” an ELISA is not guaranteed. Table 1 shows several examples of successes and failures, including detailed information on the antibody pairs tested for each target.

†Assays not verified for application to serum samples.
*For human IgA, both antibodies in the pair are sold under one catalog number, with no clone identification provided.
Conclusion
Lumit® technology has enabled the development of fast and easy bioluminescent immunoassays for analyte detection. While complete assay systems, as well as useful application notes, are available for a number of targets, the Lumit® platform also provides powerful DIY tools to enable researchers to build novel Lumit® Immunoassays for their targets of interest. Given the ease of antibody testing with Lumit® Immunoassays as described here, and the substantial performance and workflow benefits of a Lumit® Immunoassay, adapting an assay to the Lumit® format (or “Lumitizing” an assay) can yield significant productivity gains, including for screening applications.
References
- Hwang, B. et al. (2023) Lumit: A Homogeneous Bioluminescent Immunoassay for Detecting Diverse Analytes and Intracellular Protein Targets. In: Matson, R.S. (eds) ELISA Methods in Molecular Biology, vol. 2612. Humana, New York, NY. 195–224. https://doi.org/10.1007/978-1-0716- 2903-1_15
- Dixon, A. S. et al. (2016) NanoLuc Complementation Reporter Optimized for Accurate Measurement of Protein Interactions in Cells. ACS Chem. Biol. 11, 400–8. https://doi.org/10.1021/ acschembio.5b00753
- O’Brien, M. (2021) Lumit® Cytokine Assays: Interpolating Data with the GloMax® Discover. Promega Notes
More Information
To learn more about our Lumit immunoassay products, visit our Lumit Technology Page.
Related Resources

Webinar: Lumit™ Immunoassays
This webinar covers how Lumit™ Immunoassays are a faster method for analyte detection.
Article: Lumit™ Cytokine Assays
Learn how to Interpolate Data with the GloMax® Discover.