HiBiT: A Tiny Tag Expanding Endogenous Protein Detection
By Johanna Lee • January 30, 2024
So you’re obsessed with a protein. You want to know what it does, where it goes and what it interacts with. You even want to control how it behaves. But there’s a problem. It’s so rare you can’t recognize it from the crowd of background proteins. And the Western blot takes so long your spouse wonders why you never make it home for dinner. Did I mention you can’t even find a good antibody? Well, there might be a better way.

Scientists at Promega have developed a new way to tag endogenous proteins. The tag is called HiBiT—a small 11 amino acid peptide that binds with high affinity to another larger subunit called LgBiT. The bound complex has luciferase activity, and will release luminescent signal in the presence of added furimazine substrate. In a recent study published in ACS Chem. Biol., Promega scientist Marie Schwinn and colleagues tagged several endogenous proteins with HiBiT and quantified their levels and modification in response to various stimuli. They showed that HiBiT is highly quantitative, extremely sensitive and much faster than conventional immunoassays.
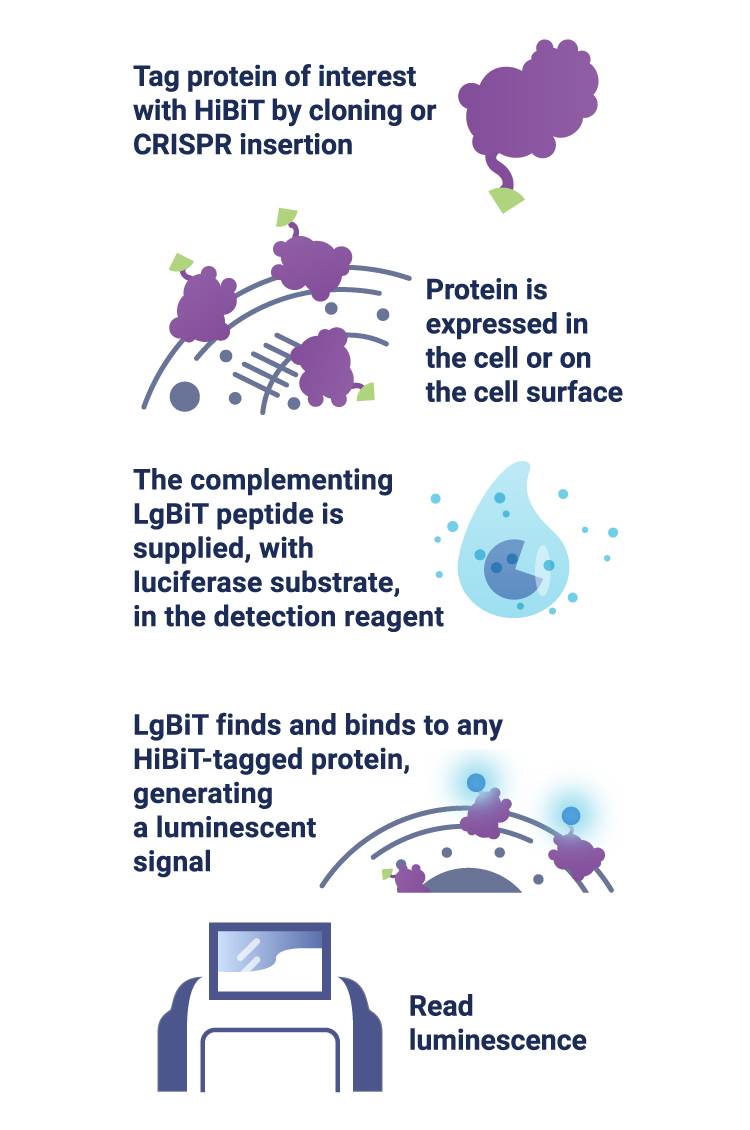
Detect low-abundance proteins
Some proteins are everywhere, but others are rare. Many low-abundance proteins play important roles in cell function, but are difficult to detect and quantify. The HiBiT assay is based on bioluminescence, which is more sensitive than fluorophore-based methods, such as GFP. This allows detection at extremely low levels. Not only is it sensitive, it is also highly quantitative. Serial dilution of purified HiBiT-tagged protein produced a broad linear range of at least 7 orders of magnitude. Luminescent signal could be detected at less than 1 amol (Schwinn et al., 2017).
Get higher insertion rates
How do you stalk an endogenous protein? You tag it. The easiest way to do this nowadays is to use CRISPR/Cas9 genome editing to insert a reporter, like GFP or luciferase, into the endogenous loci. These big reporters generally require plasmid donors, which have lower insertion efficiencies. HiBiT, on the other hand, is tiny. Because of its small size, it only needs a single-stranded oligodeoxynucleotide (ssODN) donor, which has much higher insertion rates. Schwinn et al. successfully inserted HiBiT tag to the C-terminus of endogenous HIF1α (a low-abundance transcription factor involved in cell survival and tumor growth) and various HIF1α downstream targets in HeLa cells.
No cloning, no waiting
HiBiT tagging is highly efficient and super fast. Tagging with larger reporters often requires molecular cloning procedures that can be time-consuming and soul-crushing. But because HiBiT only requires ssODN donors, all the components can be directly ordered and easily prepared. Another problem with using plasmid donors is that there might be background expression from the plasmid, which would require you to wait a few days before analysis. With HiBiT, you don’t need to wait.
HiBiT tagging is highly efficient and super fast.
HiBiT tag won’t mess up your protein
Bulky reporters interfere with your protein’s function and make them behave strangely. Because HiBiT is so small, the possibility of it affecting normal protein function is minimized.
HiBiT detection saves you time and trouble
HiBiT detection is simple: just add Nano-Glo HiBiT Lytic Detection Reagent to your cells, wait 10 minutes and pop the plate into a luminometer. The protocol is much faster than antibody-based methods since there’s no blocking, washing or antibody incubation involved. If you are interested in an orthogonal method to verify bioluminescent results, the Anti-HiBiT Monoclonal Antibody enables applications such as immunoblotting, immunofluorescence, immunoprecipitation, and fluorescence-activated cell sorting (FACS).
You can also add the highly sensitive Anti-HiBiT Monoclonal Antibody to expand the detection options for HiBiT-tagged proteins. This enables traditional antibody-based methods for detecting the HiBiT tag and removes the need for tandem-tagging approaches. Applications previously limited through bioluminescence, such as immunofluorescence (IF) are enabled through the administration of the Anti-HiBiT Monoclonal Antibody (Nagashima et al., 2023).
Easily detect endogenous proteins in primary cells
HeLa cells are great, but you also want to see your protein in a more biologically relevant environment. You’re thinking primary cells. But primary cells can be difficult to work with. They generally grow more slowly and have lower transfection/insertion efficiencies. To analyze protein abundance, you might have to sort cells and isolate clones to get a detectable signal, and some primary cells might die out before you can get a result. Isolating clones is not required for HiBiT because of its high sensitivity. Schwinn et al. edited primary human umbilical vein endothelial cells (HUVECs) to express HiBiT-tagged HIF1α. They were able to detect an increase in HIF1α-HiBiT levels after various drug treatments without isolating clones, so measurements could be made within 24–48 hours of editing.
Detect proteins in live cells in real-time
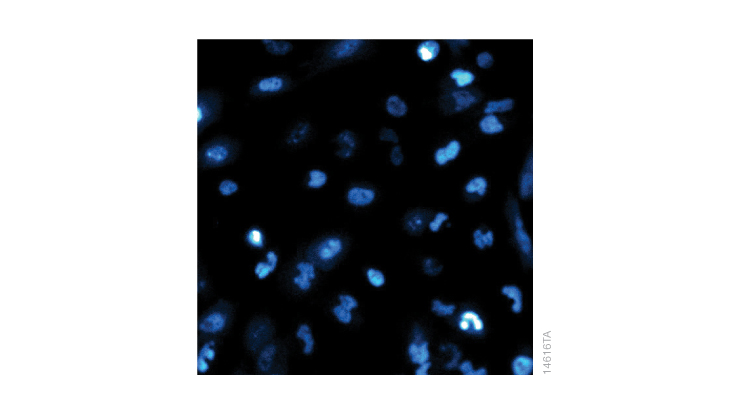
Proteins are dynamic. There is constant expression, transportation, interaction, modification and degradation in response to environmental changes—and they happen fast. The expression of HIF1α, for example, increases in hypoxic conditions, but rapidly degrades once oxygen is resupplied. Other scientists have monitored this change by preparing cells on multiple plates and lysing a different plate every five minutes, then running Western blots for all samples. With HiBiT, it is possible to monitor the same plate of live cells in real-time. Schwinn et al. monitored a single plate of HiBiT-edited cells every 30 seconds after reoxygenation and found that the half-life of HIF1α-HiBiT was less than 6 minutes. If you have access to a bioluminescence microscope, you can even see where your protein is in the cell in real-time. The image below shows the localization of HIF1α-HiBiT after phenanthroline treatment. Real-time measurement, however, requires an extra step of introducing the LgBiT subunit either by viral delivery or plasmid transfection since LgBiT cannot penetrate the cell membrane.
All in all, this study shows that HiBiT tagging is a sensitive, reliable and fast way to measure endogenous protein levels and modifications. HiBiT can be applied broadly in the study of protein dynamics—and also in the field of drug discovery. (In fact, HIF1α is a popular cancer drug target.) For drug target proteins that may not have antibodies qualified for immunoassays, HiBiT provides an excellent alternative that is compatible with high-throughput processing. If you’re obsessed with a protein and immunoassays are driving you crazy, the HiBiT tag might make your life a little easier.
Reference
Schwinn, M.K. et al. (2018) CRISPR-mediated tagging of endogenous proteins with a luminescent peptide. ACS Chem. Biol. 13(2), 467–74.
Nagashima, S. et al. (2023). Development of a HiBiT-tagged reporter hepatitis E virus and its utility as an antiviral drug screening platform. Journal of Virology, 97(9).