mRNA Therapy Solutions
We have developed a broad portfolio of products and services to support every stage of your mRNA therapeutics workflow from research and development through manufacturing. These solutions are designed to meet high quality standards, varying scale needs and regulatory requirements.
mRNA Production
Our solutions to support mRNA therapeutic manufacturing cover the complete workflow, including template plasmid DNA creation, mRNA production by in vitro transcription (IVT), and RNA purification. In addition, we offer custom and cGMP manufacturing options for those companies who wish to work with an experienced manufacturing partner.
Explore solutions for: Template DNA Production | In Vitro Transcription | mRNA Purification

Template DNA Production
The template DNA for in vitro transcription can include linearized plasmid DNA, synthesized DNA, and PCR products. Check out buffers and reagents, restriction enzymes, PCR enzymes and kits and DNA extraction products for preparation of template DNA.
Template DNA must be free of impurities, such as bacterial endotoxin, reaction components and residual contaminants. The ReliaPrep™ DNA Clean-Up and Concentration System is designed to quickly concentrate and purify dilute DNA solutions, and extract and purify DNA fragments of 100bp to 10kb. The Wizard® SV Gel and DNA Clean-Up System extracts and purifies DNA fragments from agarose gels, or directly from PCR amplification reactions.

How to produce a template for in vitro transcription.
In Vitro Transcription (IVT)
RiboMAX™ RNA production systems provide all the components and buffers needed for in vitro mRNA synthesis, including RQ1 RNase-Free DNase for digesting the template DNA after the IVT reaction.
The T7 RiboMAX™ Express Large-Scale RNA Production System is an IVT system designed for the consistent production of large amounts of RNA in a short amount of time. Milligram amounts of high-quality RNA transcripts up to 14kb have been generated using this kit.
The RiboMAX™ Large-Scale RNA Production Systems can synthesize large quantities of capped or uncapped RNA transcripts up to 23kb and incorporate modified nucleotides into transcripts.
Learn more about IVT products.

Principle of the T7 RiboMAX™ Express Large-Scale RNA Production System.

RNA yield from RiboMAX™ IVT reactions is scalable up to at least 10ml. Yield per milliliter of IVT reactions (left) and total yield (right) are shown. Each replicate RiboMAX™ reaction is represented as an individual bar. RiboMAX™ IVT reactions were prepared according to the protocol described above. 100μl, 1ml, 5ml, and 10ml reactions were prepared in duplicate with the T7 Linear Control DNA (Cat.# P141A) as template (100μg/ml of reaction). 100μl no RNA Polymerase controls were also prepared (0.1ml – NE). Transcription reactions were incubated at 37°C for 4 hours. After transcription, RiboMAX™ reactions were diluted 100X in Nuclease-Free Water and assayed using the Qubit™ RNA BR Assay (Invitrogen) with 10μl of sample.
cGMP and Animal Origin-Free IVT Reagents
Does your mRNA production work require bulk quantities, unique formulations, or tailored product sizes and formats to meet your specific needs? Learn about our raw materials for therapeutic mRNA manufacturing.

Comparison of large-scale IVT kits. The RiboMAX™ Large Scale RNA Production System-T7, HiScribe™ T7 High Yield RNA Synthesis Kit, and TranscriptAid™ T7 High Yield Transcription Kit were used to synthesize 1.8kb luciferase transcripts and 5kb run-off transcripts containing standard nucleotides (Std.), N1-methylpseudo-UTP (U), and N1-methylpseudo-UTP + 5-methyl-CTP (U+C). Transcripts were co-transcriptionally capped using CleanCap® Reagent AG (3’ OMe) according to the protocol described here. Left: Transcription products were diluted 1:100 in Nuclease-Free Water and analyzed using the RNA ScreenTape Assay. The resulting gel images are displayed for transcripts synthesized using each kit. Bands are scaled to the highest peak in each lane (scaled to sample). Right: 1.8kb transcripts were purified using a column-based RNA cleanup kit and quantified using the QuantiFluor® RNA System (Cat.# E3310). Resulting purified 1.8kb transcript concentrations and yields are displayed.
mRNA Purification
After the IVT reaction, mRNA needs to be purified from the reaction mixture so that reaction components do not inhibit downstream applications for the mRNA. The purification process also concentrates the mRNA. The ProNex® Size-Selective Purification System is a magnetic bead-based technique that can provide a simple, fast, effective and automation-friendly method for RNA purification.
Learn more about RNA extraction and nucleic acid clean-up systems.

Side-by-side comparison of two magnetic bead-based RNA purification chemistries. Fifty microliters of pre-purified RNA was purified with the ProNex® Size-Selective Purification System and a competitive kit using the manufacturer's recommended protocols.
mRNA Analytical Characterization and QC Assays
The development and manufacturing of mRNA therapeutics require rigorous analytical characterization and quality control (QC) assays to ensure the safety, efficacy, and quality of the final product. These assays are critical at various stages of the mRNA therapeutic lifecycle, from research and development to manufacturing and quality control. We offer a broad portfolio of products to support stringent measurements of mRNA quality to ensure optimal results from mRNA therapeutic development.
Explore solutions for: Impurity Detection | Quantitation | Identity Determination | Potency and Function

Impurity Detection
One common impurity in IVT reactions is a byproduct, double-stranded RNA (dsRNA). dsRNA is highly immunogenic, and it can be detected by several intracellular or endosomal sensors, leading to inflammation, translation inhibition and cell death. Therefore, it is important to detect and minimize the amount of dsRNA in mRNA produced for therapeutic uses. Existing methods to detect dsRNA in mixed solutions lack quantitation and sensitivity.
We have developed two novel assay systems for dsRNA detection and quantitation using bioluminescence: the Lumit® dsRNA Detection Assay and the TLR3 Reporter Bioassay.

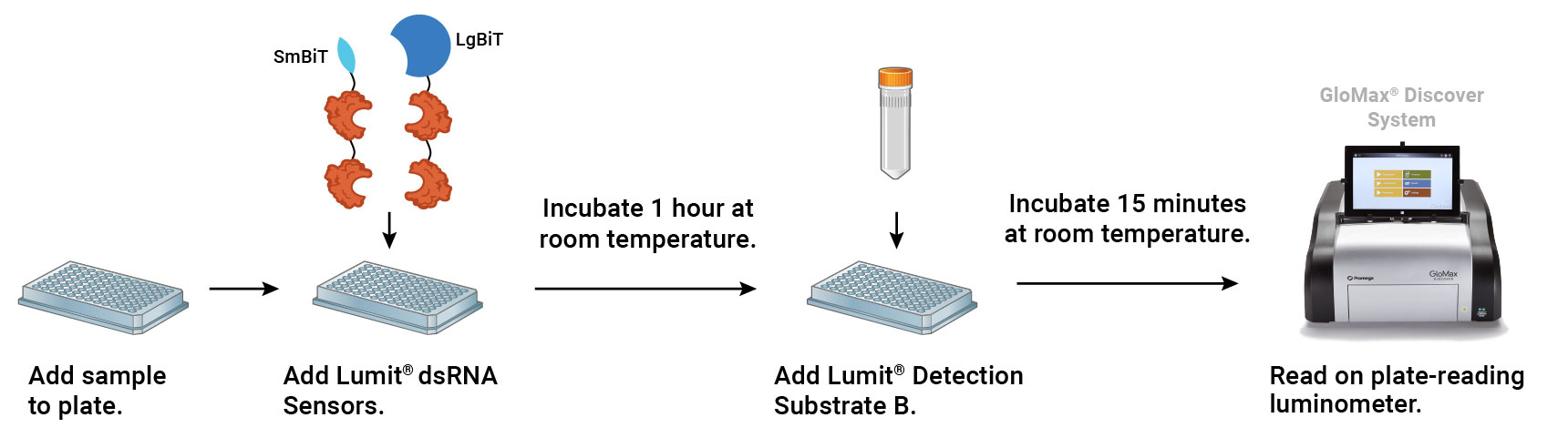
Principle of the Lumit® dsRNA Detection Assay. The assay detects dsRNA using dsRNA binding domains (DRBDs) genetically fused to SmBiT and LgBiT. Dimerization of the BiT-DRBDs on dsRNA induces complementation of NanoBiT® luciferase and generation of light.
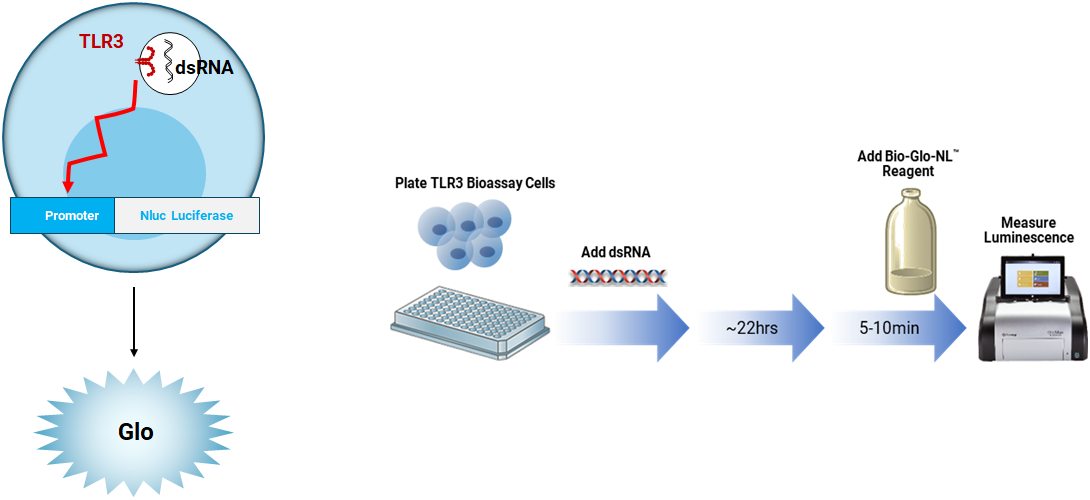
Principle of the TLR3 Bioassay. The assay measures the potency and stability of agonists designed to activate TLR signaling. Treatment with a TLR agonist results in promoter-driven luminescence that can be detected and quantified using Bio-Glo-NL™ Reagent.
Quantitation
Accurate quantitation of mRNA is important for therapeutic applications. We offer fluorescent RNA detection reagents that pair with sensitive detection instruments for single samples or microplates.
- Fluorescent RNA-binding dye for quantitation of small amounts of RNA (100pg) in solution
- Detection on microplates with the GloMax® Instruments or single tubes with the Quantus™ Fluorometer

Identity
Confirming the sequence is vital in the production of mRNA therapies and vaccines, during both the stages of template DNA creation and mRNA product identity verification. Sanger sequencing offers precision, straightforward workflows, scalability, and consistent reproducibility, which are essential for maintaining consistent testing and ensuring the safety of the end product.
The Spectrum Compact CE System is an integrated and efficient benchtop instrument that brings you the independence to perform Sanger sequencing and fragment analysis in your laboratory, under your control, and at your convenience.


In Vitro Translation
In vitro translation is often used to characterize mRNA products. Rabbit Reticulocyte Lysate System, Nuclease Treated and Wheat Germ Extract contain energy-regenerating systems that are optimized for translation and ready to use for in vitro translation of mRNAs. Both were shown to be able to generate full length proteins using capped mRNA with and without modified nucleotides.
Transcend™ tRNA is a precharged, biotin-labeled, lysine tRNA that can be used with in vitro translation systems to tag a protein for nonradioactive detection.
Learn more about in vitro translation reagents.
Functional Assays
We offer a broad portfolio of functional assays that were developed based on sensitive bioluminescence technology. These assays include luciferase reporter assays, simple no-wash immunoassays, enzyme activity and binding assays, and much more.
Learn more about functional bioassays.
Custom Potency Assay Development
Our bioassay development and services include new assay development, modification of existing assays, bioassay optimization and qualification, biologic drug profiling and custom cell manufacturing.