T Cell Therapy
Engineered T cell therapies involve the genetic modification of a patient’s own immune cells (autologous therapy) with antigen-specific T cell receptors (TCRs) or chimeric antigen receptors (CARs). When re-introduced into the patient, these precision engineered cells instruct the patient’s immune system to recognize and kill tumor cells. Future advancements in this field will include more sophisticated functional T cell engineering, novel types of cell therapies (NK and macrophage cell therapies) and the availability of off-the-shelf cell therapy products (allogeneic therapy).
The development and manufacture of functionally complex T cell therapies requires an equally sophisticated toolbox of biochemical and cell-based assays. Leveraging our extensive portfolio of bioluminescent assay technologies, we have developed a suite of assays to enable:
- Discovery of novel antigen-specific TCRs and CARs
- Measurement of T cell activation and cytokine production
- Development of target cell killing (TCK) potency assays
TCR Discovery and Characterization
Cellular T cell redirection by genetic modification of T cells with chimeric antigen receptors (CAR) or transgenic T cell receptors (TCR) is one of the important strategies employed in redirected T cell therapies, representing a new paradigm for cancer treatment. To facilitate the screening and characterization of new transgenic TCRs, we developed two TCRαβ-null reporter T cell lines, which are CD4+ or CD8+. Reintroduction of peptide-specific TCR α and β chains into TCRαβ-null reporter T cell lines results in peptide-dependent TCR activation. The select expression of CD4 or CD8 in the TCRαβ-null reporter T cell lines can enable the development of transgenic TCRs for both MHCI- and MHCII-restricted tumor antigen targets. Together, these bioluminescent bioassays represent a new set of tools for the discovery and development of T cell-based immunotherapies.
We have developed a suite of T Cell Activation Bioassays that measure TCR signaling via NFAT or IL-2 driven expression of a luciferase reporter. To better enable researchers developing engineered T cell therapies, we also created a T Cell Activation Bioassay that lacks expression of endogenous TCRαβ chains. The T Cell Activation Bioassay (TCRαβ-KO) eliminates endogenous alpha and beta chain cross-pairing with transgenic TCRs and provides an improved assay window.
- Measure activity and potency of transgenic TCRs and CARs
- Bioassay cells lack endogenous TCRαβ expression
- Available in CD4+, CD8+, CD4+CD8+, CD4-CD8- formats


Activity of transgenic TCRs measured using the T Cell Activation Bioassay (TCRαβ-KO, CD4+). A) TCRαβ-KO (CD4+) Cells were transfected with a Positive Control TCR Plasmid (or mock transfected) and then co-cultured with MHCII APC Cells (HLA-DR+ cells) and increasing concentrations of the Positive Control Peptide. B) TCR-mediated T cell activation was measured using the T Cell Activation Bioassay (endogenous TCR, CD4+) and the T Cell Activation Bioassay (TCRαβ-KO, CD4+). The T Cell Activation Bioassay cells were co-cultured with HLA-DR-positive cells and increasing amounts of cognate peptide. The modified T cells are activated once they bind antigens on tumor cells, as indicated by luminescence. The data demonstrate that the T Cell Activation Bioassay can be used to screen transgenic TCRs used for T cell immunotherapy.
T Cell Activation and Cytokine Production
Cytokine production, especially IFN-γ and IL-2, is a characteristic feature of activated cytotoxic T cells. We have developed T cell cytokine immunoassays using the novel Lumit® Immunoassay platform. These assays offer sensitive bioluminescent detection of the release of critical cytokines such as IL-2 and IFN-γ.


Lumit® immunoassays measure IL-2 and IFN-γ production from activated CD8 T cells. Purified CD8+ T cells (effector cells) were combined with Raji B cells (target cells) and a serial dilution of Blincyto® (CD3xCD19 bispecific T cell engager). IFN-γ (A) and IL-2 (B) levels were measured with the corresponding Lumit® Cytokine Immunoassays to detect cytokine release from the effector cells.
Potency Testing
To ensure the effectiveness of CAR T-cell therapy, functional potency assays are employed to evaluate the ability of CAR T cells to recognize and eliminate cancer cells. We have developed potency assays for the Drug Substance (i.e., CAR and TCR vectors and viral capsids), as well as for the Drug Product (i.e., CAR-T and TCR-T). CAR-T functional potency assays involve co-culturing CAR T cells with target cancer cells and measuring key functional outcomes, such as target cell killing. These assays play a vital role in the development, characterization and quality control of CAR T-cell therapies in oncology.
Target Cell Killing (TCK)
Targeted killing of tumor or infected cells by effector cells is the primary mechanism of action for many immunotherapy drugs. Thus, it is necessary to demonstrate robust and specific killing of target cells during drug development. Conventional cytotoxicity assays do not distinguish between target and effector cells. Further, target cell-specific assays are often laborious and require radioactive or fluorescent labeling.
The HiBiT Target Cell Killing bioassay platform is simple, homogenous, highly sensitive and provides a robust assay window. It enables highly sensitive and specific measurement of target cell killing induced by a variety of biologic drugs, including mAbs, bispecific Abs and CAR-T cells. These assays provide a choice of popular target cells expressing a HiBiT fusion protein. Upon target-cell killing, a bright luminescent signal is generated.


Extended assay incubation time reveals serial TCK activity. T cells transduced with CAR-19 or a GFP control lentivirus were combined with HiBiT Target cells (Ramos) at different effector:target (E:T) ratios. After incubation for 24 hours (A) or 48 hours (B), NanoBiT® Extracellular Detection Reagent was added, and luminescence was read on a GloMax® Discover plate reader. The EC50 shifts left over time, indicating serial TCK activity at lower E:T ratios.
In-Process Potency Testing of T Cell Redirecting Lenti-CAR Virus
For lentivirus vectors used in CAR-T manufacturing, guidance from regulatory authorities recommends additional measures of potency beyond transgene expression of the viral vector in CAR-T cells. To address this need, we developed a MoA-based potency assay to measure CAR:target cell engagement for T cell redirecting lentiviral vectors. The bioassay provides a stability indicating, quantitative luminescent readout that is readily implemented as part of your CAR-T workflow.
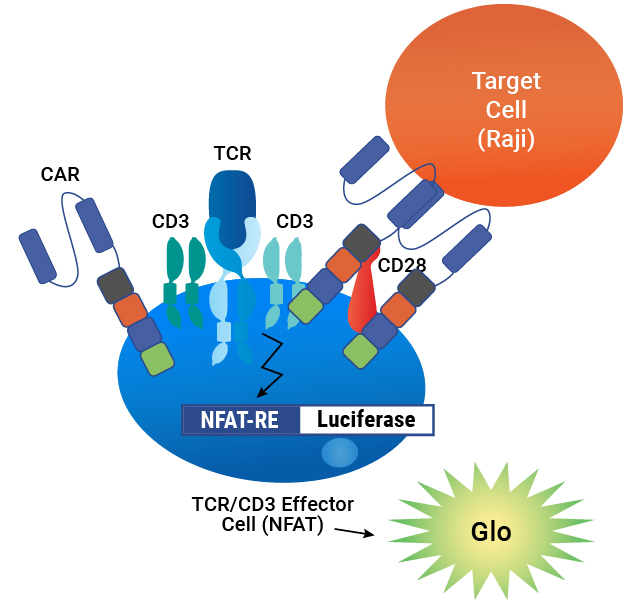
Assay principle. CAR is expressed on the surface of TCR/CD3 Effector Cells containing an NFAT-Luciferase reporter. When the CAR is engaged by an antigen on a target cell, luminescence is induced in proportion to the signaling.
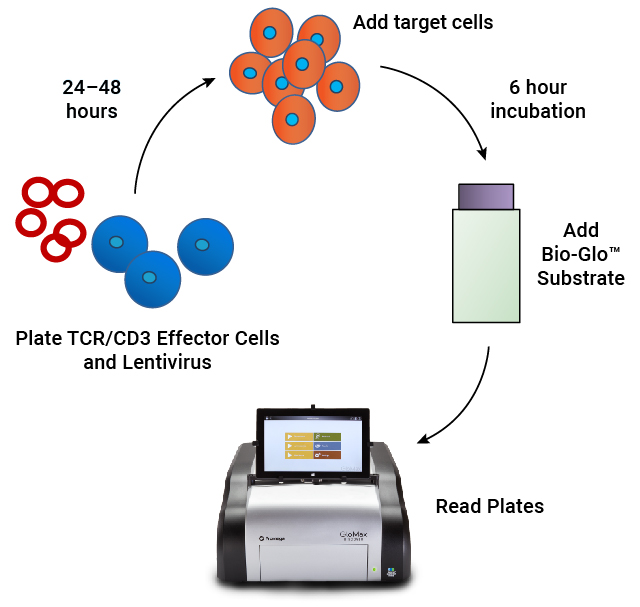
Assay workflow. TCR/CD3 Effector Cells are combined with CAR lentivirus in 96-well plates. After 24–48 hours of incubation, antigen-expressing target cells are added. Following a 6 hour induction period, Bio-Glo™ Substrate is added and luminescence is quantified.
Lentivirus Bioassay is Specific


Lentivirus Bioassay Is Stability Indicating

| Heat Treatment Time (hours) |
EC50 |
% Inhibition of Max |
|---|---|---|
| 0 |
0.81 | Reference |
| 2 |
1.0 |
26% |
| 6 |
2.4 |
18% |
| 24 | 2.5 |
47% |
| 48 | 5.0 |
67% |
Forced degradation samples of CAR-19 lentivirus were prepared by incubating at 37°C for 2–48 hours, then analyzed using TCR/CD3 Effector Cells and Raji Target Cells. The assay demonstrates a loss of lentiviral potency with heat treatment, as measured by an increase in EC50 and a decrease in the maximum response.
T Cell Metabolism
Metabolic signature is a critical determinant of T cell function and is valuable information for effective CAR-T cell therapy. Our bioluminescent cell metabolism assays can be used to measure the ability of TCR-modified or CAR-T engineered T cells to respond to metabolic demands, redox environment and nutrient availability. These plate-based assays are easily automated and well-suited for high-throughput sample analyses.

Overview of metabolism assay portfolio. These bioluminescent assays measure metabolic and oxidative stress states of donor T cells used in T cell therapy. The assays measure: metabolic cofactors—ATP, NADP; glucose metabolism—glucose uptake, lactate, glycogen; amino acid metabolism—glutamine/glutamate, BCAA; lipid metabolism—triglyceride/glycerol, cholesterol/cholesterol ester and fatty acid oxidation; and TCA cycle markers—malate, isocitrate and pyruvate.
Easy-to-Use and Sensitive Metabolite Assays

Example reaction schematic for the NAD(P)H-Glo™ Detection System. The assay is a bioluminescent, homogeneous, single-reagent-addition assay that quantitatively monitors the concentration of the reduced forms of NADH and NADPH levels in enzymatic reactions. The oxidized forms, NAD+ and NADP+, are not detected and do not interfere with luminescent signal.
Measure CAR-T Cell Metabolic Dynamics
CAR-T cell activation and proliferation trigger several metabolic changes that facilitate rapid energy generation and support the biosynthetic demands of CAR-T cells. Notably, CAR-T cells exhibit elevated glucose uptake and utilization, along with enhanced lactate production. These cells also demonstrate increased expression of key glycolytic enzymes and transporters, which enables efficient glucose metabolism.
A.

B.

C.

D.

Media metabolite dynamics vary throughout activation and expansion of T cells. (A) Cell proliferation; (B) glucose consumption; (C) glutamine consumption; and (D) lactate secretion.
T Cell Growth and Expansion
During CAR-T development, T cell expansion plays a crucial role in the generation of therapeutic products. In this process, T cells are isolated from a patient's blood and genetically modified to express a chimeric antigen receptor. These modified T cells are then cultured and stimulated with various growth factors, such as IL-2, IL-7 and IL-15, to promote their proliferation and activation. This expansion phase generates a large population of CAR-T cells that can effectively recognize and target cancer cells. Understanding the viability of the expanding CAR-T cell population is a critical factor. Our suite of cell viability assays provide a simple, add-mix-measure, homogeneous method for determining the number of viable cells in a culture by detecting and quantifying ATP, which is an indicator of metabolically active cells.
Example Workflow for CAR-T Development

A.

B.

C.

Cell viability measurement with the CellTiter-Glo® 2.0 Assay. Luminescence measured with the CellTiter-Glo® 2.0 Assay is proportional to the number of viable Jurkat cells in culture over three orders of magnitude in (A) 96-well plates, (B) 384-well plates and (C) 1536-well plates.
Cell Identity
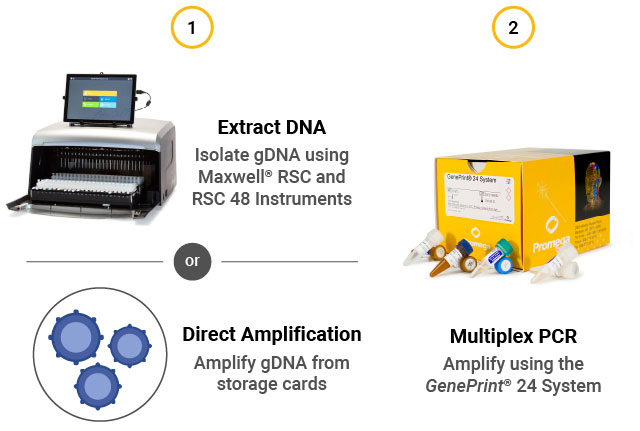
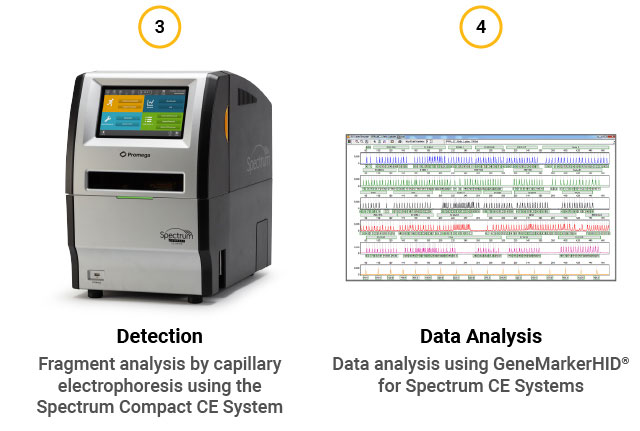
STR assay workflow. (1) Extract gDNA from cells or perform direct amplification. (2) Perform multiplex PCR using the GenePrint® 24 System. (3) Detect fragments by capillary electrophoresis using the Spectrum Compact CE System. (4) Analyze data using GeneMarkerHID® for Spectrum.
The GenePrint® 24 System can be used for STR based identification of donors in the following applications:
- CAR-T Manufacturing QC: Monitor the unique STR profile of CAR-T cells during manufacturing to confirm their identity
- Mixed Sample Analysis: Determine percentage of allogeneic CAR-T cells in a mixture of CAR-T and native cells

Concordance of STR profile data. Unmodified T cells and engineered CAR-T product were spotted on FTA cards. Direct amplification was performed using the GenePrint® 24 System and analyzed on the Spectrum Compact CE System. The results confirm 100% concordance of alleles between engineered CAR-T cells and original donor cells.
In Vivo Bioluminescence
Bioluminescence Imaging (BLI) proves to be an excellent technique for investigating CAR-T cells and tumor target cells concurrently in an animal model, offering valuable insights into the effects of CAR-T cells on tumor growth, disease processes, drug efficacy, and the evaluation of potential cell or gene-based therapies. Visualization of multiple luciferases within the same animal is possible by employing orthogonal luciferase/luciferin combinations. BLI is particularly well suited for such studies due to its ability to noninvasively track and monitor these interactions. Using BLI, researchers can gain a comprehensive understanding of how CAR-T cells function within the complex context of a living organism, providing critical information for the development and refinement of targeted cancer treatments.
- Bioluminescence requires no external light source for emission, allowing for low background and increased sensitivity compared to fluorescent reporters.
- The same reporter tool can be used for in vitro and in vivo studies, simplifying translational studies.
- Firefly luciferase (FLuc) and NanoLuc® luciferase (NLuc) provide an ideal dual-imaging solution because of substrate specificity between the two enzymes.
- The method enables monitoring of different cellular events or different cell populations to be tracked in the same animal.
- Substrate delivery occurs sequentially, allowing the initial signal to decline prior to reading the second signal.

