A New Luminescent Assay for Detection of Reactive Oxygen Species
Promega Corporation
Publication Date: 10/13
Abstract
Here we introduce the ROS-Glo™ H2O2 Assay, a rapid and sensitive luminescent assay for detection of reactive oxygen species. This homogeneous assay measures H2O2 levels directly in cell culture or in defined enzyme reactions. The assay does not use horseradish peroxidase, avoiding the high false hit rate associated with HRP-based systems, and does not require cell sample manipulation for cell-based applications. The ROS-Glo™ H2O2 Assay allows identification of conditions or test compounds such as small molecule inhibitors or inducers that alter ROS levels, is amenable to automation and can be multiplexed with other assays to generate more information from each assay well.
Assays for Reactive Oxygen Species
Reactive oxygen species (ROS) are chemically reactive molecules that contain oxygen. ROS are beneficial to the cell, having roles in cell signaling and as natural byproducts of normal metabolism (1) . ROS can also lead to cellular damage, or oxidative stress, as a result of environmental factors (e.g., radiation) or aberrant metabolism (1) (2) . ROS are reactive (e.g., superoxide, singlet oxygen, H2O2) and have a short half-life in solution. Enzymatic and non-enzymatic reactions result in conversion of ROS species to hydrogen peroxide (H2O2) in cells (3) . For example, superoxide dismutase rapidly converts superoxide to H2O2. H2O2 has a relatively long half-life in solution and can diffuse out of the cell, which makes it a good marker of oxidative stress. H2O2 is a biomarker of enzymatic activities that either consume or produce H2O2 .
Current fluorescent enzyme assay formats are prone to false hit rates that are too high for efficient screening applications. Current cell-based ROS assays require large numbers of cells with sample manipulation and are not amenable to higher throughput applications. New assays are needed in order to study the biology of ROS and to screen chemical compounds for their capacity to alter H2O2 levels in cultured cells or in enzymatic reactions. The ROS-Glo™ H2O2 Assay is a luminescent assay that detects H2O2 directly, minimizing the false hit rate. Importantly, the ROS-Glo™ Assay does not use horseradish peroxidase (HRP), which is known to cause a high number of false hits. The ROS-Glo™ Assay also provides a simple format for both cell-based and enzymatic assays.
Since various ROS are converted to H2O2 in the cell, and H2O2 is the longest-lived ROS, an increase in H2O2 can reflect a general increase in the ROS level. The ROS-Glo™ Assay uses an H2O2 substrate that directly reacts with H2O2 to produce a luciferin precursor, which is not a suitable substrate for luciferase. Addition of the ROS-Glo™ Detection Solution converts the precursor to luciferin, and provides luciferase and other components to generate a light signal proportional to the level of H2O2 present (Figure 1).
 Figure 1. ROS-Glo™ H2O2 Assay chemistry.
Figure 1. ROS-Glo™ H2O2 Assay chemistry. H2O2 Substrate is applied to samples containing H2O2, which converts it to a luciferin precursor. Upon addition of a second reagent containing Ultra-Glo™ Recombinant Luciferase, ATP and d-Cysteine, the precursor is converted to luciferin and, in the presence of Ultra-Glo™ Recombinant Luciferase, produces light.
Simple ROS Assay Format
The ROS-Glo™ Assay is designed as a single kit that will work for both biochemical and cell-based applications (Figure 2). For cell-based assays, the ROS-Glo™ H2O2 Substrate can be added to the wells of cultured cells together with test compounds or after cell treatment. The cells are then incubated under normal mammalian cell culture conditions. After incubation, ROS-Glo™ Detection Solution is added and the plate is incubated for 20 minutes prior to reading luminescence. There is no need to remove media and the assay can be performed directly in the cell culture plate.
The ROS-Glo™ H2O2 Assay can also be performed in non-lytic, cell-based mode. In this case the H2O2 Substrate is added to the reaction and allowed to react with H2O2. The media that contains the H2O2 Substrate is then removed to a separate plate for incubation with the ROS-Glo™ Detection Solution. This assay format allows the original wells to be used for another assay. Multiplexing ROS-Glo™ with other assays in this way allows more information to be gained from each well.
 Figure 2. Overview of the ROS-Glo™ H2O2 Assay protocol for cell-based or biochemical detection.
Figure 2. Overview of the ROS-Glo™ H2O2 Assay protocol for cell-based or biochemical detection. For enzymatic reactions, the H2O2 Substrate can be added directly to the enzyme reaction along with the reaction components if the enzyme reaction is performed at pH 7.5-9. In this pH range, the H2O2 Substrate reacts well with H2O2. If the enzyme reaction is performed outside of this pH range, the H2O2 Substrate can be diluted directly into 1M Tris-HCl pH 8.0 and added after the enzyme reaction is complete. The Tris buffer is both a good diluent for the H2O2 Substrate and also brings the enzyme reaction into the desired pH range, allowing the reaction between the H2O2 Substrate and H2O2 to progress.
Enzymatic Reactions
The ROS-Glo™ H2O2 Assay can be used to measure the activity of enzymes that generate or eliminate H2O2. For example, NADH oxidase is an enzyme that uses NADH and oxygen to produce H2O2 and NAD+. The luminescent signal from the ROS-Glo™ Assay correlates with the amount of H2O2 that is produced by NADH oxidase (Figure 3). Once the appropriate levels of NADH oxidase and NADH substrate are determined, the assay can be used to identify inhibitors of the enzyme in a chemical library by looking for a decrease in luminescence.
 Figure 3. NADH oxidase enzymatic activity. Increasing concentrations of NADH oxidase were incubated with NADH in enzyme reaction buffer (30mM potassium phosphate pH 7.5, 0.05% BSA) at ambient temperature for 1 hour. The H2O2 Substrate was added and the reaction incubated for 30 minutes. ROS-Glo™ Detection Solution was then added and luminescence determined on a Tecan M1000 Pro plate reader after 20 minutes incubation. The signal-to-background ratios were calculated. Alternatively, this experiment could be performed by adding the H2O2 Substrate during the enzyme reaction.
Figure 3. NADH oxidase enzymatic activity. Increasing concentrations of NADH oxidase were incubated with NADH in enzyme reaction buffer (30mM potassium phosphate pH 7.5, 0.05% BSA) at ambient temperature for 1 hour. The H2O2 Substrate was added and the reaction incubated for 30 minutes. ROS-Glo™ Detection Solution was then added and luminescence determined on a Tecan M1000 Pro plate reader after 20 minutes incubation. The signal-to-background ratios were calculated. Alternatively, this experiment could be performed by adding the H2O2 Substrate during the enzyme reaction.Cell-based Assays
The ROS-Glo™ H2O2 Assay can be used to measure an increase or decrease in ROS generated in cells. H2O2 rapidly diffuses into and out of cells and the H2O2 Substrate will react with H2O2 in each assay well, regardless of whether it was first generated in the cell or in the media. Menadione is a compound that interrupts the electron transport chain in mitochondria, producing large amounts of ROS in cultured cells. In Figure 4, K562 cells were treated with menadione and the ROS-Glo™ H2O2 Assay was used to determine ROS production. Menadione treatment resulted in a concentration-dependent ROS increase.
 Figure 4. ROS induction in cultured cells. K562 cells in 384-well plates were treated with increasing concentrations of menadione. The H2O2 Substrate was added during cell treatment. The reaction was incubated for 2 hours. An equal volume of ROS-Glo™ Detection Solution was then added and the reaction incubated for a further 20 minutes before luminescence was determined on a Tecan M1000 Pro plate reader.
Figure 4. ROS induction in cultured cells. K562 cells in 384-well plates were treated with increasing concentrations of menadione. The H2O2 Substrate was added during cell treatment. The reaction was incubated for 2 hours. An equal volume of ROS-Glo™ Detection Solution was then added and the reaction incubated for a further 20 minutes before luminescence was determined on a Tecan M1000 Pro plate reader.Small Molecule Screening
The ROS-Glo™ H2O2 Assay offers many benefits over other commercially available assays for small molecule screening, including low false hit rate, signal stability and compatibility with liquid handlers (e.g., fewer steps, no need to perform the assay in the dark). Because the H2O2 Substrate reacts directly with H2O2, the false hit rate is significantly reduced compared to other assays (e.g., Amplex® Ultra Red). Many ROS assays require HRP and result in an excessive number of false hits either through inhibition of HRP or competition with H2O2 or the reagent probe for the HRP enzyme.
We compared the ROS-Glo™ Assay to the Amplex® Ultra Red HRP-based Assay in a screen of the Library of 1280 Pharmacologically Active Compounds (LOPAC1280; Sigma-Aldrich). The assay chemistries were tested directly by determining the ability of each assay to detect 10µM H2O2 (no enzymes or cells involved). The Amplex® Ultra Red screen generated a hit rate of 7.1% while the ROS-Glo™ Assay generated a hit rate of only 0.5%. HRP reacts with numerous compounds, which appears to result in a reduction in H2O2, but in reality these are false hits. Additionally, the ROS-Glo™ Assay identified signal inducers that the Amplex® Ultra Red screen missed (Figure 5). These compounds are natural ROS generators that make H2O2 in media through redox cycling, but due to HRP inhibition the Amplex® Red assay did not detect the H2O2 generated (Figure 5 and Table 1).
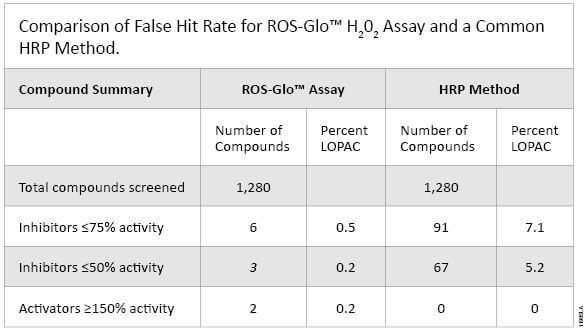 Table 1. Comparison of the False Hit Rate for ROS-Glo™ H2O2 Assay and A Common HRP Method
Table 1. Comparison of the False Hit Rate for ROS-Glo™ H2O2 Assay and A Common HRP Method
 Figure 5. ROS-Glo™ H2O2 Assay vs. HRP-based method. Both assay chemistries were screened against the LOPAC small molecule library. All compounds were tested at 10µM concentration. The assays were tested for their ability to detect 10µM H2O2. The x-axis summarizes the ROS-Glo™ H2O2 Assay and the y-axis summarizes the HRP-based (Amplex® Ultra Red) assay results.
Figure 5. ROS-Glo™ H2O2 Assay vs. HRP-based method. Both assay chemistries were screened against the LOPAC small molecule library. All compounds were tested at 10µM concentration. The assays were tested for their ability to detect 10µM H2O2. The x-axis summarizes the ROS-Glo™ H2O2 Assay and the y-axis summarizes the HRP-based (Amplex® Ultra Red) assay results.The ROS-Glo™ H2O2 Assay was also used to screen the LOPAC library using HepG2 cells in complete medium (Figure 6). The compounds that increase the ROS-Glo™ Assay signal are compounds in the LOPAC library that generate ROS in cultured mammalian cells (true hits).
 Figure 6. Cell-based small molecule screen using ROS-Glo™ H2O2 Assay. HepG2 cells (1,200/well) were plated in a 384-well, low-volume assay plate pre-loaded with 5nl LOPAC compounds (final concentration of compound was 10µM /reaction). H2O2 Substrate was added to the assay plate to a final concentration of 25µM and the reaction incubated at 37°C in 5% CO2 for 2 hours. ROS-Glo™ Detection Solution was then added and luminescence determined after a 20-minute incubation.
Figure 6. Cell-based small molecule screen using ROS-Glo™ H2O2 Assay. HepG2 cells (1,200/well) were plated in a 384-well, low-volume assay plate pre-loaded with 5nl LOPAC compounds (final concentration of compound was 10µM /reaction). H2O2 Substrate was added to the assay plate to a final concentration of 25µM and the reaction incubated at 37°C in 5% CO2 for 2 hours. ROS-Glo™ Detection Solution was then added and luminescence determined after a 20-minute incubation.Multiplexing the ROS-Glo™ H2O2 Assay
To gain more information from each assay well, the ROS-Glo™ Assay can be multiplexed with a variety of other assays using the non-lytic assay protocol provided in the Technical Manual (#TM391). The H2O2 Substrate is first incubated with the reaction of interest, then the medium or buffer containing the H2O2 Substrate is removed and added to the ROS-Glo™ Detection Solution in a new well. This protocol preserves the original assay well for further analysis with a different assay, e.g., cell viability determination with the CellTiter-Glo® Assay.
The ROS-Glo™ Assay and the CellTox™ Green Cytotoxicity Assay can also be easily performed in multiplex format to determine ROS production, cytotoxicity and total cell number (for normalization). To perform these two assays in multiplex, both the H2O2 Substrate and the CellTox™ Green dye are added to the cells during treatment. The fluorescent CellTox™ Green signal is read first, then the ROS-Glo™ Detection Solution is added to generate the luminescence signal, which correlates with ROS levels (Figure 7). The ROS-Glo™ Detection Solution also lyses the cells so another fluorescence reading of the CellTox™ Green signal will determine the total cell number and allow normalization.
 Figure 7. ROS-Glo™ H2O2 Assay multiplexed with a kinetic cytotoxicity assay (CellTox™ Green Cytotoxicity Assay).
Figure 7. ROS-Glo™ H2O2 Assay multiplexed with a kinetic cytotoxicity assay (CellTox™ Green Cytotoxicity Assay). HepG2 cells were plated at 2,000 cells/well in a 384-well plate and incubated overnight. The cells were then treated with either 100µM menadione, 100µM pyrogallol or 200µg/ml digitonin and incubated at 37°C in 5% CO2 for 2 hours. 1X CellTox™ Green Dye and 25µM H2O2 Substrate were added to the cell culture at the time of dosing. After incubation the CellTox™ Green fluorescence signal was measured on a Tecan Infinite® M1000 Pro plate reader (excitation 485nm, emission 520nm, bandwidths 5nm). An equal volume of ROS-Glo™ Detection Solution was added to the wells and incubated for 20 minutes at room temperature. Luminescence signal from the ROS-Glo™ Assay was measured on the same instrument.
Summary
The ROS-Glo™ H2O2 Assay measures changes in the level of reactive oxygen species in cultured mammalian cells. The assay can also be used to measure the activity of enzymes that generate or eliminate H2O2. This allows easier screening of chemical libraries for compounds that affect the activity of enzymes such as NADH oxidase. The ROS-Glo™ Assay technology does not rely on a reaction catalyzed by HRP, and therefore results in a much lower false hit rate. The assay can be performed easily in multi-well plates in a quick “add-mix-measure” format. The assay can also be multiplexed with other assays, allowing more data to be obtained from each assay well.
Related Products
Related Protocols
Article References
- Alfadda, A. and Sallam, R. (2012) Reactive oxygen species in health and disease. J. Biomed. Biotech. Published online, August 8. doi: 10.1155/2012/936486.
- Wittmann, C. et al. (2012) Hydrogen peroxide in inflammation: Messenger, guide, and assassin. Adv. Hematol. Published online June 12. doi: 10.1155/2012/541471.
- Newsholme, P. et al. (2012) Reactive oxygen and nitrogen species generation, antioxidant defenses and β-cell function: A critical role for amino acids. J. Endocrin. 214, 11-20.
How to Cite This Article
Scientific Style and Format, 7th edition, 2006
Duellman, S., Shultz, J., Vidugiris, G., and Cali, J. A New Luminescent Assay for Detection of Reactive Oxygen Species. [Internet] 10/13. [cited: year, month, date]. Available from: https://worldwide.promega.com/resources/pubhub/a-luminescent-assay-for-detection-of-reactive-oxygen-species/
American Medical Association, Manual of Style, 10th edition, 2007
Duellman, S., Shultz, J., Vidugiris, G., and Cali, J. A New Luminescent Assay for Detection of Reactive Oxygen Species. Promega Corporation Web site. https://worldwide.promega.com/resources/pubhub/a-luminescent-assay-for-detection-of-reactive-oxygen-species/ Updated 10/13. Accessed Month Day, Year.